
����Ŀ�����������г��ù��������������ⶨ�����̵ĺ�������Ӧԭ��Ϊ��
2Mn2++5S2O82-+8H2O![]() 2MnO4-+10SO42-+16H+
2MnO4-+10SO42-+16H+
(1)�ִ���ѧ�У�������_________�ϵ���������������Ԫ��
(2)�Դӷ��ӵ����幹�ͺ�ԭ�ӵĵ縺�ԡ�����ԭ���ϵŵ��ӶԵȽǶȽ���Ϊʲô��ˮ�ṹʮ�����Ƶ�OF2�ļ��Ժ�С��______________________��
(3)��֪H2S2O8�Ľṹ��ͼ��
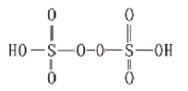
��H2S2O8��ԭ�ӵĹ���ӻ���ʽΪ________________��������Ӧ�б���ԭ��Ԫ��Ϊ_____________��
��S��̬ԭ���е��ӵĿռ��˶�״̬��________________ �֡�
��������Ӧÿ����1 mol MnO4-��S2O82-���ѵĹ��ۼ����ͼ�����ĿΪ___________��__________��
(4)һ�������£�ˮ���Ӽ��ͨ���������H2O���ӽ�ϳ���ά�Ǽܽṹ�����еĶ������Ѩ�пɰ�������С���ӣ��γ�����ˮ�ϰ����ᄃ�塣
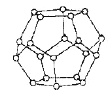
����ͼ��һ����ˮ���ӹ��ɵ���ʮ������Ǽ�(��o����ʾˮ����)��������������Ϊ__________��
��ʵ���ñ��������������Ϊ18.8kJ��mol-1���������ۻ���Ϊ5.0kJ��mol-1����ԭ�������__________��
(5)���ľ����������ǣ��������Ŀ�ܵĶ�������������ijһ������������õ��¾�����ԭ��������ھ���ѧ�ϵ���ƽ�Ƶķ�����+ (1/2��1/2��0) (C���ģ�����+ (0��1/2��1/2) (A����)����+ (1/2��0��1/2) (B����)������ƽ����ָ����֮һ��

��I2��_______���A������B����C�������ľ���.
(6)ʯī�����ɲ�״ʯī�����ӡ���ABAB��ʽ�ѻ����ɣ���ͼ��a)��ʾ����������һ��ʯī������������ͼ��b����ʾ��
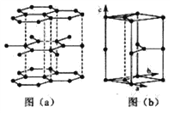
����ͼ�л���������C���ͶӰ���á��� ���̼ԭ��λ�ü��ɣ�_______________��
���̼ԭ��λ�ü��ɣ�_______________��
�ڼ���ʯī�IJ���Ϊ300 pm��C-C����Ϊ150 pm������ʯī������ܶ�Ϊ______g�� cm-3(̼Ԫ�ص��������Ϊ12��NA=6.0��1023������������һλС������
���𰸡� ԭ�ӹ��� OF2��H2O�����幹�����ƣ�ͬΪV�Σ���ˮ���ӵļ��Ժ�ǿ����OF2�ļ���ȴ��С��������Ϊ�� (1)�ӵ縺���Ͽ���������ĵ縺�Բ����������ĵ縺�Բ��2��OF2����ԭ���������Թµ��Ӷԣ������� F-O���й��õ��Ӷ�ƫ��F�������ļ��� sp3 O 9 �Ǽ��Լ���Ҽ� 2.5NA 30 Һ̬ˮ����Ȼ���ڴ������������ڻ�ʱֻ�ƻ��˲�������� B 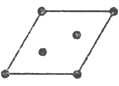 2.3
2.3
��������(1)�ִ���ѧ�У�������ԭ�ӹ����ϵ���������������Ԫ�أ��ʴ�Ϊ��ԭ�ӹ��ף�
(2)�ӵ縺���Ͽ���������ĵ縺�Դ���������ĵ縺�Բ�ֵ��OF2����ԭ���������Թµ��Ӷԣ�������F-O���й��õ��Ӷ�ƫ��F�������ļ��ԣ��Ӷ�����H2O���ӵļ��Ժ�ǿ����OF2���ӵļ���ȴ��С���ʴ�Ϊ���ӵ縺���Ͽ���������ĵ縺�Դ���������ĵ縺�Բ�ֵ��OF2����ԭ���������Թµ��Ӷԣ�������F-O���й��õ��Ӷ�ƫ��F�������ļ��ԣ��Ӷ�����H2O���ӵļ��Ժ�ǿ��
(3)��H2S2O8�У���ԭ�Ӽ۲���Ӷ���=�� �����Ӷ�+����ԭ���ϵŵ��Ӷ�=4+![]() (6-4��1-2)=4�����Բ�ȡsp3�ӻ����÷�Ӧ�У�MnԪ�صĻ��ϼ�����(+2��+7)��OԪ����-2�ۺ�-1�ۣ�����-1��ת��Ϊ-2�ۣ����ϼ۽��ͣ����Ա���ԭ��Ԫ��ΪO���ʴ�Ϊ��sp3�ӻ���O��
(6-4��1-2)=4�����Բ�ȡsp3�ӻ����÷�Ӧ�У�MnԪ�صĻ��ϼ�����(+2��+7)��OԪ����-2�ۺ�-1�ۣ�����-1��ת��Ϊ-2�ۣ����ϼ۽��ͣ����Ա���ԭ��Ԫ��ΪO���ʴ�Ϊ��sp3�ӻ���O��
��S��̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p4������3p4��4������ռ��3����������ӵĿռ��˶�״̬�У�1s��2s��2px��2py��2pz��3s��3px��3py��3pz����9�֣��ʴ�Ϊ��9��
���ɷ�Ӧ��֪��MnԪ�صĻ��ϼ�����(+2��+7)��OԪ�صĻ��ϼ۽���(-1��-2)������10molSO42-ת�Ƶ���10mol���ӣ���ÿ����1 mol MnO4-��ת�Ƶ���5mol���ӣ�S2O8 2-����2.5mol(��2.5NA)O-O��Ǽ��Թ��ۼ����ʴ�Ϊ���Ǽ��Լ���2.5NA��
(4)���ɴ˽ṹ��֪���˵�Ԫ�к���ˮ���ӵĸ���Ϊ��20������ÿ��ˮ�����γɵ��������2����Ԫ������ÿ��ˮ�����γ��������Ϊ�� ![]() �����ܹ��γ������Ϊ��20��
�����ܹ��γ������Ϊ��20��![]() =30���ʴ�Ϊ��30��
=30���ʴ�Ϊ��30��
�ڱ��������������Ϊ18.8kJmol-1�������ۻ���Ϊ5.0kJmol-1��˵�����ۻ�ΪҺ̬ˮʱֻ���ƻ���һ�������������Һ̬ˮ������������ʴ�Ϊ��Һ̬ˮ����Ȼ���ڴ��������
(5)���ľ����������ǣ��������Ŀ�ܵĶ�������������ijһ������������õ��¾�����ԭ�������˵��������İ���ԭ����������ԭ����������ƽ�����IJ�����þ����ǽṹ��Ԫ��2���塣A��B��C��������ķ�����һ�µ�(�ο�ͼ�е�����ϵ)����ֻ�н�������ܵĶ�������ac������ģ������¾�������ԭ�������������bc���Ļ�ab���ģ��õ����¾����е�ԭ�����겻ͬ��ԭ��������˲�����A��Cƽ�ƣ��ʵ���B���ģ���ѡB��
(6)�ٸ���ͼ(b)��֪����C���ͶӰ�����Կ����ı��ε��ĸ������ϸ���1��̼ԭ�ӣ����ͼ(a)����ͼΪ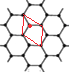 ������һ����һ��̼ԭ��λ�ڸ�ԭ�ӵĶԳ�λ�ã������C���ͶӰΪ
������һ����һ��̼ԭ��λ�ڸ�ԭ�ӵĶԳ�λ�ã������C���ͶӰΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
����ʯī������ÿ��̼ԭ������������������������ÿ����������������̼ԭ�ӣ�����ÿ��̼ԭ��ʵ��ռ�е������ε���ĿΪ0��5����12g̼����1mol̼ԭ�ӣ����Ժ���0.5mol�����Σ���ʯī�����У�ÿ������������12��![]() =2��̼ԭ�ӣ��������������Ϊ
=2��̼ԭ�ӣ��������������Ϊ![]() 1502��300��1030cm3����������
1502��300��1030cm3����������![]() ���Լ�����ܶ�Ϊ
���Լ�����ܶ�Ϊ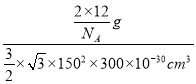 =
=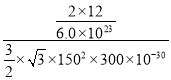 g/cm3=2.3g/cm3���ʴ�Ϊ��/span>2.3��
g/cm3=2.3g/cm3���ʴ�Ϊ��/span>2.3��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����
��������ˮ������һ��ʱ��pH��С
��Al��Fe�������������ܵ�����Ĥ�����ڲ����������ʴ
��Al2O3��Fe2O3��Ϊ������������Զ�����������
��pH��5.6��7.0֮��Ľ�ˮͨ����Ϊ����
A. �٢� B. �ڢ� C. �٢� D. �ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ϩ�IJ����Ǻ���һ������ʯ�ͻ�����չˮƽ����Ҫ��־֮һ������˵����ȷ����(����)
A. ��ϩ�����Ҵ�����������Ӧ
B. ��ϩ��ʹ����KMnO4��Һ��ɫ
C. һ����������ϩ�ɷ����ۺϷ�Ӧ
D. һ����������ϩ�ɷ����ӳɷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������������������ȫȼ����������������(����)
A.CH4B.C2H6C.C3H8D.C6H14
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��230Th��232Th���ʵ�����ͬλ�أ�232Th����ת����233U�������й�Th��˵����ȷ����
A. Th Ԫ�ص���������232 B. Th Ԫ�ص����ԭ��������231
C. 232Th ת����233U�ǻ�ѧ�仯 D. 230Th��232Th�Ļ�ѧ������ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A(C2H4)�ǻ������л�����ԭ�ϡ���A�ͳ������л���ɺϳ�һ���������Ϻ�һ����ȩ�����ϣ�����ϳ�·����ͼ��ʾ�����ַ�Ӧ������ȥ����

��֪��

�ش��������⣺
��1��B�ķ���ʽ��___________��C�к��еĹ����������� ____________��
��2����DΪ��ȡ�������廯��������������Ʒ�Ӧ��ÿ��D������ֻ����1����ԭ�ӣ�D����Ԫ�ص���������ԼΪ13.1%����D�Ľṹ��ʽΪ___________���ķ�Ӧ������________________��
��3���ݱ�������Ӧ�����������£���NaHSO4��H2OΪ�������У���д���˷�Ӧ�Ļ�ѧ����ʽ��____________________________________��
��4����д���������������ı���ȩ������ͬ���칹��Ľṹ��ʽ��___________________��
i .���б�����![]() �ṹ
�ṹ
ii.�˴Ź���������4��壬�ҷ����֮��Ϊ3��2��2��1
��5����������EΪ�����ѵ�ͬϵ�����Է��������ȱ����Ѵ�14������ʹFeCl3��Һ��ɫ��E������ͬ���칹�干��(�����������칹)________________�֡�
��6������ �ĺϳ�·�ߣ�д����2-�ȱ���ͱ�Ҫ�����Լ��Ʊ�
�ĺϳ�·�ߣ�д����2-�ȱ���ͱ�Ҫ�����Լ��Ʊ� �ĺϳ�����ͼ��_______________________________________
�ĺϳ�����ͼ��_______________________________________
�ϳ�����ͼʾ�����£�CH2 = CH2![]() CH3CH2Br
CH3CH2Br![]() CH3CH2OH
CH3CH2OH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з����У�ֻ��������û���������ǣ�������
A.N2B.CH4C.CH2=CH2D.CH��CH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ˮ�����౦�����Ȼ��Դ����ͼ��ij�����Ժ�ˮ��Դ�ۺ����õIJ�������ʾ��ͼ��


��1�����оٺ�ˮ���������ַ���__________��__________��
��2�����Ƶ�ʳ��ˮͨ�����ӽ���Ĥ���۵�⡣��ͼΪ���ӽ���Ĥ�����ԭ��ʾ��ͼ����ⱥ��ʳ��ˮ�����ӷ���ʽΪ______________________���Ը��ݵ缫��Ӧ����������Ĥ������X��__________��
��3����֪[Ca(OH)2=5.5��l0-6��Mg(OH)2=1.8��l0-11]����c(Mg2-)=3.6��l0-3mol/L �� MgCl2��Һ�� cmol/L��NaOH��Һ�������ϣ�Ҫʹ���Һ����Mg(OH)2������c���ٵ���__________mol/L������������õ�Mg(OH)2�����г�����Ca(OH)2��������ȥ��д����ķ�����_____________________________��
��4����ҵ��ȡþ������MgCO3�����ȷֽ⣬�ʶ����ӽ�̿�����Ȼ��õ�MgCl2�����ͨ�����MgCl2��ȡMg��д���÷��ʶ����Ļ�ѧ����ʽ__________________________________________����������Ȼ�þ���õ�þ������ȴ��Ϊ����þ�����������У�þ�������������Χ����ȴ����__________��ѡ������ĸ����
A��Cl2 B��N2 C��H2 D������
��5����ͬѧ��Ϊ������ۺ�ɼ���Mg(OH)2�����õ�MgO���ٵ�����ڵ�MgO�ƽ���þ�������ɼ�ʵ�鲽�裬����ʵ��ļ�Լ��ԭ���Ҳ�ͬ��Ĺ۵㣬����������__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼ��ʾ��ʵ��װ���У���A��B��C���ֱ�Ϊ���²�ͬ��ϵ�����ʱ���ش��й����⣺
(1).��AΪ���ᡢBΪʯ��ʯ��CΪ��������Һʱ��С�Թ��е�������__________________________��С�Թ��з�����Ӧ�Ļ�ѧ����ʽ��___________________________________________������ʵ��ɵó��Ľ�����_______________________��
(2).��AΪ����ʳ��ˮ��BΪ��ʯ��CΪ����KMnO4��Һʱ��С�Թ��е�������______________��A��B��Ӧ�ķ���ʽ��____________________________��
(3).װ����D���ֵ�������____________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com