
����Ŀ���������Ժʿ����Ժʿѧ��ͬ���ġ����ӹ��������ն�����������ˣ�ȫ��������������˵��BF3��TiO2��CH3COOH��CO2��NO����ï����NH3��HCN��H2S��O3�������ϩ���Ƶ��ڶ������ӹ������е����ǡ�
(1)д��Fe2���ĺ�������Ų�ʽ________________________________��
(2)����˵����ȷ����________��
a��H2S��O3���Ӷ���ֱ����
b��BF3��NH3��������
c��CO2��HCN���ӵĽṹʽ�ֱ���O=C=O��H��C��N
d��CH3COOH������̼ԭ�ӵ��ӻ���ʽ�У�sp2��sp3
(3)TiO2����Ȼ�����У����ȶ���һ�־���ṹ��ͼ�� �����ʾ________ԭ�ӡ�
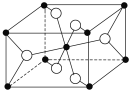
(4)����(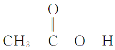 )�۷е�ܸߣ������ڴ����Է��Ӽ�����ϵĶ�����(��һ����״�ṹ)���뻭���ö�����Ľṹ��_________________________________________��
)�۷е�ܸߣ������ڴ����Է��Ӽ�����ϵĶ�����(��һ����״�ṹ)���뻭���ö�����Ľṹ��_________________________________________��
���𰸡�1s22s22p63s23p63d6��[Ar]3d6 cd ��(O) 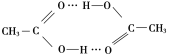
��������
(1)����26��Ԫ��,��ԭ�Ӻ�����26�����ӣ���ԭ��ʧȥ2�����ӱ�ΪFe2+,���ݹ���ԭ��֪�������Ӻ�������Ų�ʽΪ1s22s22p63s23p63d6��[Ar]3d6��
�ʴ�Ϊ��1s22s22p63s23p63d6��[Ar]3d6��
(2)a.H2S��O3���Ӷ���V�Σ� a�����
b.BF3Ϊƽ�������Σ�NH3��Ϊ�����Σ�b�����
c.CO2��HCN���ӵĽṹʽ�ֱ��ǣ�O=C=O��HC��N��c����ȷ��
d. CH3COOH�����м���-CH3����ԭ���γ�4���������ӻ������ĿΪ4�����õ���sp3�ӻ����Ȼ���-COOH����̼ԭ���γ�3���������ӻ������ĿΪ3�����õ���sp2�ӻ���d����ȷ��
�ʴ�Ϊ��c d
(3) �������=![]() ,�������=
,�������=![]() ,������ͺ��������Ϊ2:1,���ݶ�������Ļ�ѧʽ֪,�����ʾ��ԭ��.
,������ͺ��������Ϊ2:1,���ݶ�������Ļ�ѧʽ֪,�����ʾ��ԭ��.
�ʴ�Ϊ����(O)
(4)����������Ӽ���,�Ȼ��ϵ���ԭ������һ�����������̼��˫������ԭ���γ����,���Ըö�����ĽṹΪ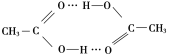 ��
��
�ʴ�Ϊ��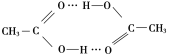 ��
��


 ������ѧ���̲���ȫ���ϵ�д�
������ѧ���̲���ȫ���ϵ�д� ������ʱ����ҵ����ϵ�д�
������ʱ����ҵ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�ij����������Һ�п��ܴ��ڣ�Fe2+��Cu2+��Mg2+��SO32-��Br-��SO42-�������е�һ�ֻ��֣��ֽ�������ʵ�飺
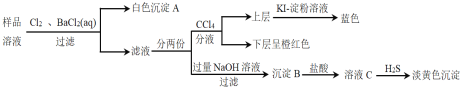
����˵������ȷ���ǣ�
A.��Һ��һ��������Mg2+��Cu2+B.��Һ��һ��������SO32-
C.��Һ��һ������Fe2+��Br-��SO42-D.����B�п��ܺ���������þ���϶�������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
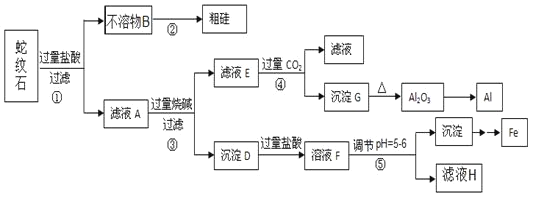
����Ŀ������ʯ����Կ�����MgO��![]() ��
��![]() ��
��![]() ��ɡ�ijʵ��С����������������ֱ��Ƶõ���Al��Fe��Mg��Si��
��ɡ�ijʵ��С����������������ֱ��Ƶõ���Al��Fe��Mg��Si��
�й��������������pH���±���
���������� | �������↑ʼ����ʱ��pH | ����������ȫ����ʱ��pH |
| 1.9 | 3.2 |
| 9.4 | 11.6 |
��1������ʯ������������þ�������⼸��Ԫ�صļ����ӵİ뾶��С�����˳��_____����ҺA�к��е��������� _________��
��2����Ԫ�������ڱ��е�λ��______��������̼�ĵ���ʽ___________��
��3���������з�Ӧ�Ļ�ѧ����ʽΪ ____�������������ɳ���G�����ӷ���ʽΪ _____��
��4���������е���pH��![]() ʱ�������õ����Լ�
ʱ�������õ����Լ�![]() �����
�����![]() ____________��
____________��
a��NaOH b����ˮ c��MgO d��Mg��OH��2
��5������ҺH��ȡ����Mg���������£�
![]()
������ұ��þ�ķ�����__________���ڸ����HCl�����м���MgCl2��6H2O��ȡ��ˮ�Ȼ�þ��ԭ����__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������(Na2FeO4)��һ�ָ�Ч�����͵�ˮ���������ڼ��������½��ȶ��������Ի�������������ת��ΪFe(OH)3��O2���ױ�H2��ԭ����ҵ���Ի�����(��Ҫ�ɷ�ΪFeS2����������NiS��CuS��SiO2������)Ϊԭ���Ʊ�Na2FeO4������ijЩ������Դ�Ĺ���������ͼ��
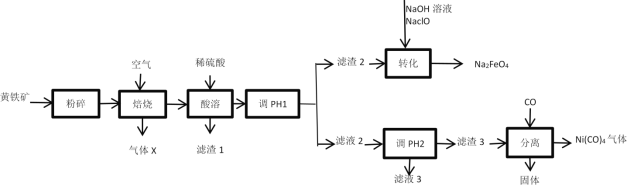
��֪��
��.�����������ʯ�еĽ���Ԫ�ؾ������������ʽ���ڡ�
��.��Һ������Ũ��С��1��10-5mol/Lʱ�������ӳ�����ȫ��
��1������������Ŀ����___��
��2������XΪ__������1Ϊ__��
��3��������������pH1����ʵ�����Ŀ����___��
��4����ת���������з�������Ҫ��Ӧ�����ӷ���ʽΪ___��
��5�����õ�ⷨ�Ʊ�Na2FeO4�Ĺ���ԭ����ͼ��ʾ��
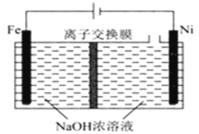
����װ�ù���ʱ�������ĵ缫��ӦʽΪ___��
�����ӽ���ĤӦѡ��___(����������������)���ӽ���Ĥ��ԭ��____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵������ȷ����
A.0.1mol��L1NaOH��Һ�ӳ������µ�60�棬��pH��С
B.��Ũ�ȵİ�ˮ��NaOH��Һ����ϡ���������ԣ���c(NH4+)=c(Na+)
C.��Ũ�ȵİ�ˮ��NaOH��Һϡ����ͬ�ı�����pH���ߴ�
D.���������pH�İ�ˮ��NaOH��Һ����������AlCl3��Һ����������������ǰ�ߴ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��![]() �������г��������ʣ���ش��������⣺
�������г��������ʣ���ش��������⣺
(1)д��![]() ��ˮ��Һ�е���ķ���ʽ��______________��
��ˮ��Һ�е���ķ���ʽ��______________��
(2)![]() �����������������������е�________________________��
�����������������������е�________________________��
(3)��д��![]() ��Һ��
��Һ��![]() ��Һ��Ӧ�����ӷ���ʽ��___________________________��
��Һ��Ӧ�����ӷ���ʽ��___________________________��
��д��![]() ��Һ��
��Һ��![]() ��Һ��Ӧ�����ӷ���ʽ��_____________________________________��
��Һ��Ӧ�����ӷ���ʽ��_____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)����500mLŨ��Ϊ![]() ��Һ�����ȡ
��Һ�����ȡ![]() ����____g��Ȼ���ټ�������ˮ�������������ܽ⣬��__________����ת�Ƶ�______�У����ݣ�ҡ�ȡ�
����____g��Ȼ���ټ�������ˮ�������������ܽ⣬��__________����ת�Ƶ�______�У����ݣ�ҡ�ȡ�
(2)��ʵ��(1)�У�������������ȷ��������ʱ���ӿ̶��ߣ��ᵼ�������Ƶ���Һ�����ʵ����ʵ���Ũ��_____��������������С������������������ͬ��![]() ��
��
(3)��ʵ��(1)�У�ת��NaOH��Һ������ƿ��ʱ�����������������Ƶ���Һ�����ʵ����ʵ���Ũ��______![]() ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������п�����̵���(��Ҫ�ɷ�ΪZnO����������Fe2O3��CuO��SiO2��MnO��)Ϊԭ�Ͽ���������п����(ZnC2O4��2H2O)��
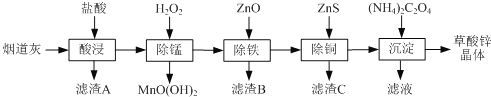
�й��������↑ʼ�����ͳ�����ȫ��pH���±���
�������� | Fe(OH)3 | Fe(OH)2 | Cu(OH)2 | Zn(OH)2 |
��ʼ������pH | 1.5 | 6.5 | 4.2 | 5.4 |
������ȫ��pH | 3.3 | 9.7 | 6.7 | 8.2 |
���ʴ��������⣺
��1������B����Ҫ�ɷ�Ϊ________ ��
��2�����̹����в���MnO(OH)2���������ӷ���ʽΪ________��
��3���ٳ���(����Cu2�����ܱ���ȥ)ʱ����ZnO���Ʒ�ӦҺpH�ķ�ΧΪ___________��
�����������г������ͭ��˳���ܵߵ�����������ʻ��С����ԭ����________��
��4�����������̲���Na2C2O4�������茶�����������п�������ļ��Ϸ�ʽ��________��
��5��������п������ȷֽ�ɵõ�һ�����ײ��ϡ����ȹ����й�����������¶ȵı仯��ͼ��ʾ��300 �桫460 �淶Χ�ڣ�������Ӧ�Ļ�ѧ����ʽΪ________��
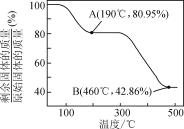
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Fe��������̼����������U�ι��е�ⱥ��ʳ��ˮʱ������˵��������ǣ� ��
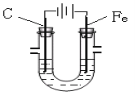
A.�����ɵõ�����
B.���������ķ�Ӧ�ǣ�2H++2e��= H2��
C.������Һ���к��ɫ��������
D.������������Һ�еμӷ�̪ʱ����Һ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com