
7. As is natural, a man as old as he ______ be very forgetful.
A. can B. must C. should D. would
6. ---- You know that you were driving at 100 kilometers per hour, don’t you?
---- No, officer, I _____ have been. This car can’t do more than 80.
A. mustn’t B. shouldn’t C. may not D. couldn’t
5. I _____ you would come today. You _____ me a phone call in advance.
A. don’t expect; should have given
B. didn’t expect; could have given
C. hadn’t expected; ought to give
D. didn’t expect; can have given
4. ---- May I speak to the patient now?
---- No, you _____ . He is too weak now.
A. needn’t B. may not C. mustn’t D. oughtn’t
3. He hurt his leg climbing the mountain; otherwise, he _____ the meeting.
A. will attend B. had attended
C. would have attended D. would attend
2. ---- Isn’t it surprising that such a good football team _____ have failed to enter the World Cup Final?
---- Yes, anything is possible on the football field.
A. would B. might C. must D. should
1. ---- Why am I so slow at doing the cloze test?
---- I guess you didn’t realize the use _____ the contexts.
A. you should have made of
B. you must have made from
C. of which you could have from
D. out of which you need make
22.(09年广东理基·28)下列说法正确的是
A.MgSO4晶体中只存在离子键
B.含Ca2+、Mg2+的水都是硬水
C.雾是气溶胶,在阳光下可观察到丁达尔效应
D.玻璃是氧化物,成分可表示为Na2O·CaO·6SiO2
答案:C
解析:MgSO4晶体中除存在离子键外,还在SO42-离子中存在共价键,故A选项错误。硬水是指含有Ca2+、Mg2+较多的水,因此B选项错误。雾是是一种胶体,属于气溶胶,因此在阳光下可观察到丁达尔效应,C选项正确。玻璃是一种硅酸盐制品,虽然可以用氧化物的形式表示为Na2O·CaO·6SiO2,但是并不说明就是氧化物,故D选项错误。
23 .(09年天津理综·1)化学与生活密切相关,下列有关说法正确的是
.(09年天津理综·1)化学与生活密切相关,下列有关说法正确的是
 A.维生素C具有还原性,在人体内起抗氧化作用
A.维生素C具有还原性,在人体内起抗氧化作用
 B.糖类、蛋白质、油脂属于天然高分子化合物
B.糖类、蛋白质、油脂属于天然高分子化合物
 C.煤经气化和液化两个物理变化过程,可变为清洁能源
C.煤经气化和液化两个物理变化过程,可变为清洁能源
 D.制作航天服的聚酯纤维和用于光缆通信的光导纤维都是新型无机非金属材料
D.制作航天服的聚酯纤维和用于光缆通信的光导纤维都是新型无机非金属材料
答案:A
解析:还原性的物质有抗氧化作用,A项正确;B项,糖类不属于高分子,错;C项,煤气化属于化学变化,错;D项,聚酯纤维属于有机物,错。
21.(09年广东理基·19)下列化学用语使用不正确的是
A.Na+的结构示意图
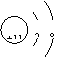

B.纯碱的化学式为Na2CO3
C.聚乙烯的结构简式为CH2=CH2
D.高氯酸(HCIO4)中氯元素的化合价为+7
答案:C
解析:聚乙烯的结构简式应为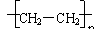
 ,CH2=CH2
,CH2=CH2 表示的聚乙烯的单体乙烯的结构简式,
表示的聚乙烯的单体乙烯的结构简式,
20.(09年广东化学·16)磷钨酸H3PW12O40等杂多酸可代替浓硫酸用于乙酸乙酯的制备。下列说法不正确的是
A.H3PW12O40在该酯化反应中其催化作用
B.杂多酸盐Na2HPW12O40与Na3PW12O40都是强电解质
C.H3PW12O40、KH2PW12O40与Na3PW12O40中都有相同的原子团
D.硅钨酸H4 SiW12O40也是一种杂多酸,其中W的化合价为+8
答案:CD
解析:因浓硫酸在制备乙酸乙酯中起到催化剂和吸水剂的作用,又杂多酸可代替浓硫酸制备乙酸乙酯,所以杂多酸同样起到催化作用,A正确;因大多数盐属于强电解质,所以杂多酸盐为强电解质,所以杂多酸盐也为强电解质,B正确;磷钨酸又可将其视作为H3PO4与WO3的混合酸,因磷酸为三元中强酸,磷酸盐中含有的酸根不同即原子团不同,C错误;从化合价和代数为0可知W的价态为+6价,D错误。
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com