
��6�֣�ij�о���ѧϰС������������װ�ý������������ȡ��̽���������������ա�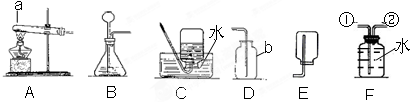
��1��д��ͼ������b������ b ��
��2��ʵ������ȡ�����Ļ�ѧ����ʽ�� ������������ ��Ӧѡ������巢��װ���� ��
��3��ʵ���Ҳ��ü��ȸ�����ع�����ȡ��������Ӧ�Ļ�ѧ����ʽ�� ������������������ ��
����ʢ��ˮ��Fװ���ռ�������Ӧ�ӵ��� ���������������� ����ٻ�ڣ�ͨ�롣
��4��������һ����ɫ����ζ��������ˮ�����壬ʵ�����ü�����ˮ�����ƺͼ�ʯ�ҵĹ�������ķ�����ȡ���飬��ʵ������ȡ���ռ�����Ӧѡ���װ������������������� ��
��1������ƿ ��2��Zn + H2SO4 ="==" ZnSO4 + H2�� B
��3��2KMnO4��K2MnO4 + MnO2 + O2�� �� ��4��AC��AE
���������������1������������ʶ��
��2��ʵ������ȡ������п��ϡ���ᷴӦ�������Ļ�ѧ����ʽ�ǣ�Zn + H2SO4 ="==" ZnSO4 + H2�������巢��װ�õ�ѡ�����ݣ���Ӧ���״̬�ͷ�Ӧ���������ڷ�Ӧ���ǹ����Һ�巴Ӧ�����ڳ����½��У�����Ӧѡ������巢��װ����B
��3��ʵ���Ҳ��ü��ȸ�����ع�����ȡ��������Ӧ�Ļ�ѧ����ʽ�ǣ�2KMnO4��K2MnO4 + MnO2 + O2��������ʢ��ˮ��Fװ���ռ�������Ӧ�ӵ��ܢ�ͨ��
��4�������վ�װ�õ�ѡ�����ݣ�ȡ��������ܶȺ��ܽ��ԣ�����������ˮ���ܶȱȿ���С�����壬����ʵ������ȡ���ռ�����Ӧѡ���װ�������AC��AE
���㣺����������ʶ�ǣ���ȡ���巢�����ռ�װ�õ�ѡ������


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��9�֣���ͼ��ʵ������ȡ�����һЩװ�ã���ͼ�ش��й����⡣
��1��д��ָ�����������ƣ���_________________����_________________��
��2��д��һ�����л�ѧ����װ��C������Ļ�ѧ����ʽ_________________����װ�����Թܿ���������б��ԭ����__________________________________��
��3��ʵ�����ڳ������ÿ�״��ʯ��CaC2����ˮ��Ӧ��ȡ������ˮ����Ȳ���壨C2H2�����������������ƣ�д���÷�Ӧ�Ļ�ѧ����ʽ_________________���÷�Ӧ�����ϸ���Ƽ�ˮ�ٶȣ�������ҷ�Ӧ����������װ��ը�ѡ�����Ϊ��ͼ�����ʺ���ȡ��Ȳ����ķ�Ӧװ����________������ĸ�����������ͼ��ʾװ���ռ���Ȳ������Ӧ��________���a����b�����˹ܿ�ͨ�롣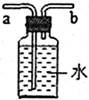
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijͬѧ���á�����ŵ���ơ�ЧӦ����������ʵ��װ�ã��뿴ͼ�ش����⣺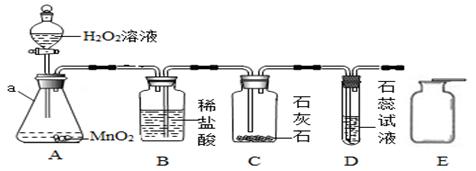
��1������a�������ǣ� ��
��2����A�з�Һ©���Ļ�����A�з�����Ӧ�Ļ�ѧ����ʽ�� ��
������D�е��ܿ�������ð��������Һ����ɫ���ɫ����B��Ӧ������������ ��
��3��д��D�з�����Ӧ�Ļ�ѧ����ʽ�� ��
��4����Eװ�����ռ�ʣ������壬������ͼ�и�Eװ�����ӵ��ܡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ������ȡ����ʱ��Ҫ��һЩװ����ͼ��ʾ����ش��������⣺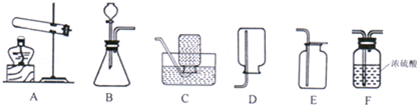
��1��ʵ��������Aװ����ȡ�����Ļ�ѧ����ʽΪ�� ����ѡ����ռ�װ���� ����Ҫ�õ��������������ѡ�������˳��Ϊ ��
��2��ijͬѧ��������Bװ�ã��ñ��Ǻ����ᷴӦ������һ����ɫ���壬�������ʵ�鷽����Ӧ�û�ѧ�����������ɵ�����Ϊ������̼����Ҫ��д���������̡�ʹ���Լ���ʵ������ �� �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(5��) ʯ��ͬѧҪ��ʵ�������Ʊ������������̼��������ͼ�ش��������⣺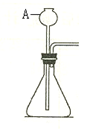
��1��д������A�ڴ�ʵ���е���; ��
��2�������ʯ��ͬѧ��ʵ�����Ʊ������������̼��ʵ��װ��ͼ����������
��3��ʵ���Ҽ��������̼�Ļ�ѧ����ʽΪ ��
��4��������̼����������ˮ���ռ���ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��4�֣����������װ�ûش����⣺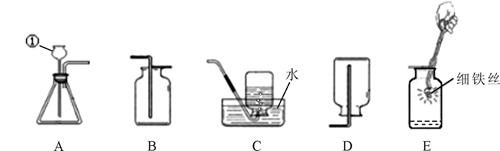
��1��д�������ٵ����� ��
��2��ʵ������ȡO2��CO2����������Aװ�ã��ռ�O2��CO2���������� װ�ã�����ĸ��ţ���
��3���ռ�һƿ��������Eʵ��ʹ�á�E�з�����ѧ��Ӧ�Ļ��������� ��
E�м���ƿ�ײ�����ˮ�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(9�֣�������ͼ�ش�����: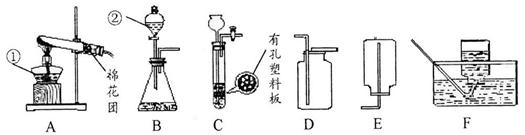
��1��д��ͼ���б�����������ƣ��� ���� ��
��2���ü��ȸ�����صķ�����ȡ������Ӧѡ�õķ���װ�ú��ռ�װ���ǣ�����ĸ��ţ�
_ ��_ ��д���÷�Ӧ��ѧ����ʽ ��
��3��д��ʵ������ʯ��ʯ��ϡ������ȡCO2�ķ�Ӧ��ѧ����ʽ_ ��
��4��ʵ������п����ϡ���ᷴӦ��ȡ��������ѡ��װ��B��װ��C��C��B��Ƚϣ����ŵ��� _ ��ѡ�õ��ռ�װ��Ϊ������ĸ��ţ�_ ��
��5��������ijͬѧ��Ƶij�ȥC0�е�C02���ռ�һƿC0��װ��ͼ��ƿ��ΪNaOHŨ��Һ���� �����������װ����_ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ͼ����ʾװ�ûش��������⡣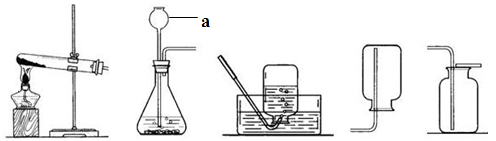
A B C D E��
��1��ͼ������a������ ��
��2���ô���ʯ��ϡ������ȡ������̼ʱ����ѡ�õķ���װ�ú��ռ�װ���� ������ĸ����ͬ������д���˷�Ӧ�Ļ�ѧ����ʽ ��
��3���ø��������ȡ����ʱ��װ��A����Ҫ����һ��Ķ��� ��Ҫ�ռ�һƿ�ϴ���������Ӧѡ�õ�װ���� ��
��4��ʵ������ȡ������ѡ�õķ���װ�ú��ռ�װ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ṩ�������н���ѡ������װ������ȡ�����װ�ã���ش��й����⣮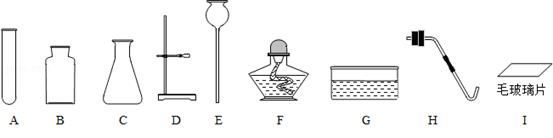
��1������C���������� ����
��2����ĥɰ�������Ӳ��������ܷ��Ե�һ�ִ������ա������������У��õ���ĥɰ�����մ��������� ��������ĸ��ţ���
��3��ʵ����������װʱ��Ϊʹ�������ܽ��ײ��������Ĵ�ʩ�� ��
������װ�����Լ�ǰ�ز����ٵ�һ������� ��
��4����װ��һ����������ڶ������̴�����ȡ�����ķ���װ�ã���Ҫ�õ��������У�����ĸ��ţ� ����д���÷�Ӧ�Ļ�ѧ����ʽ�� ����
��5��ʵ������ȡ�����һ��˼·�У�����Ҫѡ���ʵ��ķ�Ӧԭ��������������Ӧ���ܲ�����������ʵ������ȡ����ʱ���ѡ��Ӧ ������ţ�����ѡ��������Ӧ�������� ��
��NH4Cl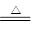 NH3����HCl��
NH3����HCl��
��NH4HCO3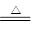 NH3����H2O��CO2��
NH3����H2O��CO2��
��2NH4Cl��Ca��OH��2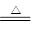 CaCl2��2NH3����2H2O
CaCl2��2NH3����2H2O
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com