
����Ŀ����������ȤС��ͬѧ�Ա�¶�ڿ������������ƹ����̽������ش��������⣺
[�������1]�� ���������ƹ�����û�б����أ�
[����ʵ��1]����ͬѧȡ�����������Թ�����������ˮ�ܽ⣬���������� BaCl2 ��Һ���۲쵽_________________��֤���������ƹ����Ѿ����� Na2CO3��
[�������2]��
��γ�ȥ�������ƹ����е����ʣ��õ����������������أ�
[����ʵ��2]����ͬѧ�Ը��������ƹ�������ᴿ�������²������̽���ʵ�飮
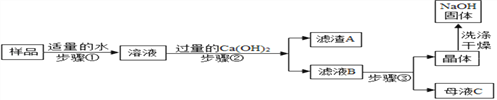
[ʵ�����]��
�Ų���ڷ�Ӧ�Ļ�ѧ����ʽ��____________������ڵIJ����н�����ˣ������������_______��
��֤����������Ѵﵽ Ca(OH)2��Һ������Ŀ�ģ����з����п��е���_________��
a��ȡ������Һ B��������ͨ������̼���壬��Һ�����
b��ȡ������Һ B���������ټ�����ʯ��ˮ���ް�ɫ����
c��ȡ������Һ B���������ټ�����Ũ̼������Һ���а�ɫ����
��Ϊ��ô������������Ʋ�����������ľ������������Ũ����_________�����ˣ� A�������ᾧB�����½ᾧ
�ȱ�ͬѧ��Ϊ��ͬѧ�����е� Ca(OH)2 ��Һ�������Թ����� Ba(OH)2 ��Һ���棬��������Ҫ�ŵ���_______________��
A��������������ˮ����������������ˮ��������������Һ���Լ�����Һ��ˮ���������������Ũ����Ч�ʣ�����Լ��Դ��
B��ֻ�������������ܳ�ȥ����
[ʵ����չ]����βⶨ���õ��ռ���Ʒ���������Ƶ����������أ�
��һƿ���õ��ռ��г�ȡ�� 20g�������ʣ������������Ϊ 19g����ȫ��������ˮ ����� 100g ��Ʒ��Һ����ȡһ�����ʵ������������Ȼ�����Һ����Ʒ��Һ��ϣ���ַ� Ӧ��õ������ʾ�����ݣ�
��Ŀ�ʹ��� | �� 1 �� | �� 2 �� | �� 3 �� | �� 4 �� |
��Ʒ��Һ����(g) | 10 | 20 | 30 | 40 |
�Ȼ�����Һ����(g) | 10 | 15 | 15 | 30 |
��������������(g) | 1.97 | 3.94 | 3.94 | X |
�ɱ��е�_________��ǡ����ȫ��Ӧ��
���������Ʒ���������Ƶ���������Ϊ_____________��(��д����������)
���𰸡� ������ɫ���� Ca(OH)2+Na2CO3===CaCO3��+2NaOH ʹ̼������ȫ��Ӧ ac B A 2��4 44.2%
��������[����ʵ��1]����ͬѧȡ�����������Թ�����������ˮ�ܽ⣬���������� BaCl2 ��Һ���۲쵽������ɫ������֤���������ƹ����Ѿ�����Na2CO3��[ʵ�����]��(1)������У�̼���ƺ��������Ʒ�Ӧ����̼��Ƴ������������ƣ���Ӧ�Ļ�ѧ����ʽ�ǣ�Na2CO3+Ca(OH)2�TCaCO3��+2NaOH������ڵIJ����н��� ���ˣ������������ʹ̼������ȫ��Ӧ��(2)a��ȡ������Һ B��������ͨ������̼���壬��Һ����ǣ�˵������������Һ������b��ȡ������Һ B���������ټ�����ʯ��ˮ���ް�ɫ���ǣ�����˵������������Һ������Ҳ������ǡ����ȫ��Ӧ��c��ȡ������Һ B���������ټ�����Ũ̼������Һ���а�ɫ���ǣ�˵������������Һ������(3)Ϊ��ô������������Ʋ�����������ľ������������Ũ�������½ᾧ��������(4)��ͬѧ��Ϊ��ͬѧ�����е� Ca(OH)2��Һ�������Թ����� Ba(OH)2 ��Һ���棬��������Ҫ�ŵ���������������ˮ����������������ˮ��������������Һ���Լ�����Һ��ˮ���������������Ũ����Ч�ʣ�����Լ��Դ��(5)�ɵ�2�κ͵�3�ο�֪����3������Ʒ��Һ�������ɵ�1�κ͵�2�����ݿ�֪����1���Ȼ�����Һ��������˱��е�2��ǡ����ȫ��Ӧ������Ϊ��4�κ͵�2��������Һ��������ȣ���˵�4��Ҳǡ����ȫ��Ӧ��(6)��Ϊ��2��ǡ����ȫ��Ӧ������Ե�2�����ݽ��м��㣺��20g��Ʒ��Һ��̼��������Ϊx��
BaCl2+Na2CO3�TBaCO3��+2NaCl
106 197
x 3.94g
![]()
x=2.12g��
20g��Ʒ��Һ����Ʒ����Ϊ��20g��![]() ��100%=3.8g��
��100%=3.8g��
��Ʒ���������Ƶ���������Ϊ��![]() ��100%=44.2%��
��100%=44.2%��


 ��������ϵ�д�
��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ʵ�����С����ͬѧ������ͼ��ʾװ�ã����������ã���ҩƷ��ȡ���л�ѧ�������岢��֤���й�����
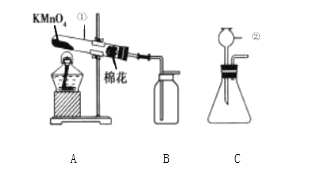
��1��д��ͼ�б�����ŵ������ڵ����ƣ�________________
��2����ȼ A �еľƾ�����ȡ���ռ������� ��װ�� A ��ȡ������ѧ����ʽΪ______________������ B ���������ռ����ķ�����_____________________
��3������ȼ��������뼯�������ļ���ƿ�У��ɹ۲쵽 �� ��ƿ������ˮ�����֣�֤������ȼ����ˮ���ɡ����ţ�������Ӽ���ƿ��ȡ����___________________����ʵ�鲽�������֤������ȼ�ջ������˶�����̼��
��4��С��ͬѧ����װ��C��һ�����Դ���_______________����װ�� C ���������������ҩƷ�����ֿ�ʼ��ȡ��������ѧ����ʽΪ________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
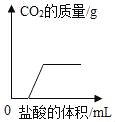
����Ŀ����ͼ�Ǽס������ֹ������ʵ��ܽ�����ߣ�
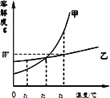
��1��t1��ʱ�����ܽ��____�ҵ��ܽ�ȣ�ѡ����ڡ������ڡ���С�ڡ�����
��2��Ҫʹ�ӽ����͵ļ���Һ��Ϊ������Һ���ɲ�ȡ�ķ�����_________________��ֻ��һ�֣���
��3���ס��Ҹ�Wg�ֱ���뵽��ֻʢ��100gˮ���ձ��У���ֽ��裬��t3��ʱ��������ҺΪ______��Һ��ѡ����͡������͡����������¶ȶ����͵�t2�棬����Һ�����ʵ���������______����Һ�����ʵ�����������ѡ����ڡ������ڡ���С�ڡ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͬѧ��ѧϰ��������̼ʵ������ȡ���о�������ʱ��̽���˶�����̼���ռ�������
���������ϣ�
(1)ͨ��״���� 1 ���ˮԼ�ܽ� 1 ���������̼��������Һ�� pH ԼΪ 5.6��pH ԽС������ ��̼��ˮ���ܽ�Խ�ࡣ
(2)ʯ��ʯ�е����ʼȲ������ᷴӦ��Ҳ������ˮ��
�����������������̼�ܲ�������ˮ���ռ���
�����ʵ�飩 ʵ��һ����ͨ��״�������ⶨ������̼����ˮ������Һ�� pH���ж϶�����̼ ��ˮ���ܽ�����������ͼ��
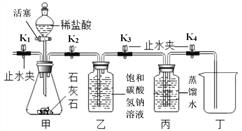
(1)��װ���з�Ӧ�Ļ�ѧ����ʽ_____��
(2)����װ�������Եķ����ǹرջ�����ֹˮ�� K1 ���� K2��K3��K4 ˫����ס��ƿ�� �۲쵽_____��˵��װ��������������
(3)ʵ��ʱ�����Ƚ���װ���еĿ����ž���������Ǵ�ֹˮ�� K1,�ر�ֹˮ�� K2 ���� �������μ�ϡ�����������ž�����������ž��ķ����� _____��
(4)�ر� K1 ���� K2 ��K3 ��K4 ������װ�����ռ���ƿ����ʱ���ر� K2 ��K3 ��K4 ���� ����װ�����ֱ��ñ�����ƿװ������Һ�� pH Ϊ 5.5 �� 6.5��������֪���ܽ�Ķ�����̼�����װ����_____(������������������������С����)��װ������װ�õ������� _____ ��
(5)ʵ���������װ�ü�Ӧ����Һ�����ʳ����Ȼ��ƻ���û�����������������ʵ��֤����IJ���(д��ʵ�鷽��������ͽ���)______
(6)ʵ������ö�����̼���ִ���������ռ��������ж�����̼�����������ͼ���ռ��� �����ж�����̼���������ʱ��仯�Ĺ�ϵͼ,��ͼ���ܵ�Ϣ��
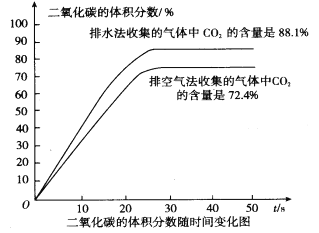
�� ________________________________________
��___________________________________________________
�����۷�˼����ʵ��һ�����������Ľ�ʵ��װ�ã�������̼������ˮ���ռ����ܽ��Ͷ��� ��̼��ˮ���ܽ��ԵĴ�ʩ��_____��_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������ֽ����Ⱦ��ϡ�ͼ���ҽҩƷ�ȵ���Ҫԭ�ϡ�ij�����ƴ�Ʒ�к����������Ȼ��ơ��Ȼ�þ��ʵ���ҽ����ᴿ���������£�
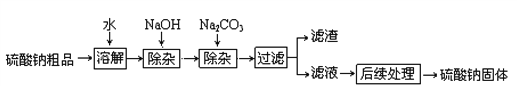
��1��������������Ҫ�ɷֵĻ�ѧʽΪ ________��____________��
��2��NaOH��Na2CO3������ӹ����ˣ��ɼ�������__________��Һ��ȥ��
��3������������������Ҫ������������Ϊ�˻�ô����������ƣ�Ӧ������______ѡ����ĸ����
A����ȫ����ʱֹͣ����
B�����ʱֹͣ���ȣ�������������
C���д�����������ʱֹͣ���ȣ�������ȥʣ���������Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��E��F��G��HΪ���������ʣ�����B��E��G���ڵ��ʣ�H����̼�����ϵ����壬C���ɵؿ��к�������Ԫ����ɵĵ��ʣ���ͼ������֮����ת����ϵ�����ַ�Ӧ������P��Ӧ������ȥ��
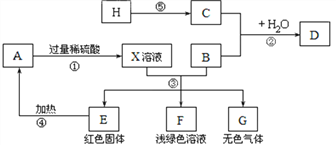
��ش�
(1)����D��Ҫ�ɷֵĻ�ѧʽΪ_______����ɫ����G������______�� X��Һ�������е�����Ϊ_______(д��ѧʽ)��
(2)д����Ӧ���������ݵĻ�ѧ����ʽ����______����_____����______��
(3)д����Ӧ�������ɺ�ɫ����Ļ�ѧ����ʽ_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ȼ�����һ�ֿ������ؽ����Σ��㷺Ӧ���ڻ��������ö���ʯ(��Ҫ�ɷ�ΪBaCO3)�Ʊ��Ȼ������幤���������£�
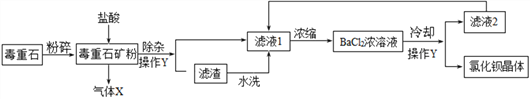
(1)����Y��������_______����Һ2�п�ѭ�����õ����ʳ�ˮ���______��
(2)ϴ���Ȼ������������˵�ϴ�Ӽ���_______(����ĸ���)��
A��20��ˮ�� ��B��20�汥���Ȼ�����Һ�� ��C��20�汥���Ȼ�����Һ
(3)����25g����ʯ(��Ҫ�ɷ�ΪBaCO3���ʲ�����ˮҲ��������)��Ʒ��100g������������Ϊ7.3����ϡ����ǡ����ȫ��Ӧ����ͨ�����㣺�ö���ʯ��̼�ᱵ������������_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼ������ȷ��ӳ���Ӧ��ϵ����
A. ��һ������NaOH��Na2CO3���Һ�м������������
B. ��һ������HC1��CuCl2���Һ�м���������Ũ����������Һ
C. ��һ������CuSO4��Na2SO4���Һ�м������������
D. ��һ����pH=l��ϡ������Һ�в��ϼ�ˮϡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������м�����Ҫ����;��
(һ)���Ĺ㷺Ӧ��
(1)���Ǻܻ��õĽ����������������Ĵ���ȴ��ʴ����ԭ����________________________��ʹ�����ƴ���ʹ������������������������¼��������˵������֢״���������������ָ_____________��
A. ���� B. Ԫ�� C. ԭ�� D. ����
(2)���Ļ�ѧ���ʱȽϻ��ã�����������������Һ��Ӧ����ƫ������(��ѧʽΪNaAlO2)��һ�ֿ�ȼ�����壬д���÷�Ӧ�Ļ�ѧ����ʽ________________________��
(��)������茶�����ȡ��̽��
���������һ����;�㷺�ĺ����������ij������(��Ҫ�ɷ�Al2O3��������SiO2��Fe2O3����)Ϊԭ����������茶��壨(NH4)aAlb(SO4)c��xH2O�����������£�
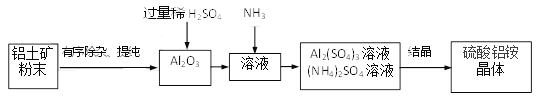
(3)�й��������̵�˵����ȷ����______��
a.��������ӡ��ᴿ�������ȼӹ���ϡ�����ٹ��ˣ���������Ŀ���dz�ȥSiO2
b. ϡ���������Ŀ��ֻ�DZ�֤Al2O3�ܽ���ȫ
(��)������茶���ֽ�Ķ���̽��
��������ȷֽ�ɵõ���������������ȤС�����ʵ��̽��������茶������ȷֽ�IJ��
(4)����ͬѧ�������ͼ1��ʾʵ��װ�ã�
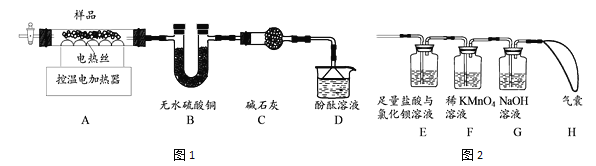
�� ʵ���У�װ��B�й۲쵽��������_________________��
�� װ��D�й۲쵽��̪��Һ���ɫ���ɴ˿�֪�ֽ�IJ�������______(д��ѧʽ)��
(5)����ͬѧ��Ϊ������茶������ȷֽ�IJ����л���SO2��SO3��N2��Ϊ������֤���ü���ʵ���е�װ��A����ͼ2��ʾװ�����ӽ���ʵ�顣�۲쵽װ��E��______��֤������SO3���ɣ���Fװ����_____________��H����û���ʹ���û��SO2��N2���ɡ�
(��)������茶���ɷֵĶ���̽��
(6)Ϊȷ��������茶������ɣ���������ʵ�飺
��ʵ��1����ȡ45.3g��Ʒ�������Ȼ�����Һ��ַ�Ӧ�����ɰ�ɫ����46.6g��
��ʵ��2����ȡ45.3g��Ʒ�ڿ����г������ȣ��ⶨʣ������������¶ȱ仯��������ͼ��ʾ��
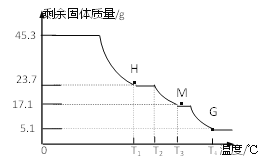
������H��T1��պ���ȫʧȥ�ᾧˮ�IJ����ᾧˮ�ĸ���x��_____________��
��T3��ʱ����M�Ļ�ѧʽ_____________��
����д��T2����T4��η�����Ӧ���ܻ�ѧ����ʽ��________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com