
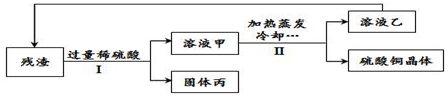
| A�� | II�к����˲�����I�в��� | |
| B�� | ��Һ����Һ���о���ͭԪ�� | |
| C�� | �����Ĵ��������У�H2SO4��CuSO4ʵ����ѭ������ | |
| D�� | ������·��Ŀɻ������������У����ڸ��ϲ��ϵ������� |
���� A�����ݾ���I����������õ��˹����Һ����з�����
B��������Һ����Һ���о�������ͭ���з�����
C������ϡ������������ܽ�����������ͭ��Һ�������ٴνᾧ���з�����
D������������������ϳɲ���֮һ���з�����
��� �⣺A������I����������õ��˹����Һ�壬����I��������ж��й��ˣ���A����
B����Һ����Һ���о�������ͭ��������Һ����Һ���о���ͭԪ�أ���B��ȷ��
C��ϡ������������ܽ�����������ͭ��Һ�������ٴνᾧ����C��ȷ��
D��������������ϳɲ���֮һ�������ڸ��ϲ��ϣ���D����
��ѡ��BC��
���� �ڽ������ʱ�����ȷ������п�������⣬Ȼ����ѧ����֪ʶ��������������ʾ���н��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| ���� | ���� | �����ʵ��Լ��ͷ��� | |
| A | CO2 | CO | ������NaOH��Һ���գ����� |
| B | NaOH | Na2CO3 | ��ϡ���ᣬ���� |
| C | N2 | O2 | ͨ�����ȵ�ͭ�� |
| D | FeCl2��Һ | CuCl2 | �������ۣ����� |
| A�� | A�� | B�� | B�� | C�� | C�� | D�� | D�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ý�����þ�������� | B�� | �����ˮ������ͭ����ʯ�� | ||
| C�� | ����������ʯ�͡����� | D�� | �Σ��ռ���̼��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
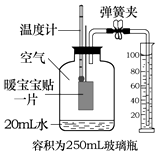 ů����������Ҫ�ɷ�Ϊ���ۡ�ľ̿��ʳ�Σ���������Դ�����۵�����ʱ��Ӧ�������������⣮С��ͬѧ���ʹ��ů���������ⶨ�����������ĺ�����ʵ�鿪ʼǰ��װ����ͼ��ʾ��ʵ������Ͳ�����벣��ƿ���ݻ�Ϊ250mL���е�ˮ�����Ϊ45mL�������������ĵ�ˮ���Բ��ƣ�������˵��������ǣ�������
ů����������Ҫ�ɷ�Ϊ���ۡ�ľ̿��ʳ�Σ���������Դ�����۵�����ʱ��Ӧ�������������⣮С��ͬѧ���ʹ��ů���������ⶨ�����������ĺ�����ʵ�鿪ʼǰ��װ����ͼ��ʾ��ʵ������Ͳ�����벣��ƿ���ݻ�Ϊ250mL���е�ˮ�����Ϊ45mL�������������ĵ�ˮ���Բ��ƣ�������˵��������ǣ�������| A�� | ʵ��ǰ������װ�õ������� | |
| B�� | ����ʵ�����ݲ�ÿ������������������Ϊ18% | |
| C�� | ��ʵ���ÿ����������������ƫ�ߣ�������ů��������ʹ���������� | |
| D�� | ������¶ȼƵĶ����ָ���ʵ��ǰ���¶Ⱥ���ܼ�¼��Ͳ��ʣ��ˮ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ���� | �� | �� | �� | �� | �� | �� | �� | �� | �� |
| ������������ | 2% | 2% | 2% | 6% | 6% | 6%] | 10% | 10% | 10% |
| ˮ���¶ȣ��棩 | 20 | 40 | 60 | 20 | 50 | 60 | 20 | 40 | 60 |
| ��ҺpH | 10.90 | 11.18 | 11.26 | 11.08 | 11.27 | 11.30 | 11.22 | 11.46 | 11.50 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 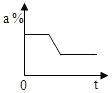 | B�� | 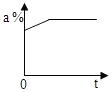 | C�� | 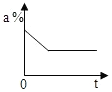 | D�� | 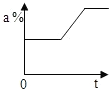 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com