
 ��ǰ��������۵ļ۸����Ԫ���ϰ�Ԫ���ȣ�������ۡ��ָж�û�����Բ��죮��ô��������ۺ���������۵ijɷ��кβ�ͬ�أ��ʹ����⣬ʵ��С��ͬѧչ��̽����
��ǰ��������۵ļ۸����Ԫ���ϰ�Ԫ���ȣ�������ۡ��ָж�û�����Բ��죮��ô��������ۺ���������۵ijɷ��кβ�ͬ�أ��ʹ����⣬ʵ��С��ͬѧչ��̽����| ʵ������ | ���� | ���� |
| ��1���ֱ�������������ۺ���������۷����Թ��У�������ˮ������һ��ʱ��μӷ�̪��Һ�� | ��������۵��ϲ���Һ��죬��������۵���Һû�б�ɫ | ����ٳ��� |
| ��2���ֱ�������������ۺ���������۷����Թ��У�������ˮ������Һ�м���Ũ���ᣬ���ȣ� | ��������۵���Һ�л�ɫ���֣��ֲ���ڣ���������۵���Һû���������� | ����ڳ��� |
| ��������� | ��������� | |
| ����۵����� | 100g | 100g |
| ������������� | 460.0g | 501.3g |
| �ձ����������ʵ������� | 520.0g | 557.7g |
���� �������е�֪ʶ���з�������̪��Һ�ڼ�����Һ�гʺ�ɫ��̼��ƺ��������ƾ����������ᷢ����ѧ��Ӧ��Ҫ����̼��Ƶĺ�������Ҫ��ȥ�������ƣ��ݴ˽�ɣ�
��� �⣺������룺�ٸ�����������۵ļӹ�����֪�����ӹ���������Ҫʹ���������ƣ�������������к����������ƣ�����������ƣ�
ʵ��̽������1����������������к����������ƣ��ʼ��ԣ���ʹ��̪��Һ��죬�����̪��Һ��
��2�����������к��еİ�������Ũ������Ȼ��ƻ��ڣ�����������۲����д����ʣ��ʿ��Բ��ü���Ũ����ķ������������Һ�м���Ũ���
��3��������������к��е��������ƻ������ᷢ����Ӧ���ʲ���ֱ�Ӽ���ó��������������У�������к��е���������Ҳ�������ᷴӦ��
�ڸ��ݱ����ṩ�����ݿ��Կ�������������۲���������̼������Ϊ��100g+501.3g-557.7g=43.6g
�躬̼��Ƶ�����Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 43.6g
$\frac{100}{x}$=$\frac{44}{43.6g}$
x��99.1g
̼��Ƶĺ����ǣ�$\frac{99.1g}{100g}$��100%=99.1%��
���99.1��
ʵ�鷴˼��ͨ��һ������۵�ȡ����������������֤�����Ը������ʵ����ʲ�����У��������Ƶ�ˮ��Һ�ʼ��ԣ���ʹ��̪��Һ��죬����������Ũ����������»��ƣ�Ȼ������̼�������������������м���ȷ��̼��Ƶĺ���������ֱ�ȡ100g��������ۺ���������ۣ�����������ˮ�ܽ⣬���ˣ�����Һ�ֳ����ݣ�������һ�ݼ���Ũ���ᣬ��һ�ݼ����̪��Һ���������м���14.6%��ϡ������ǡ����ȫ��Ӧ���ⶨ������������ձ���ʣ�����ʵ��������������������𰸿ɵ÷֣�
���� ���⿼���˶����ʳɷֵ��ƶϣ���ɴ��⣬�����������е�֪ʶ�������ṩ����Ϣ�����ʵ����ʽ��У�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ˮ����������������ɵ� | |
| B�� | ��Դ�������������������������� | |
| C�� | ��������������������������Ϊ1��2 | |
| D�� | �����������������ʹ�����ǵ�ľ����ȼ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �ŷɼ�ʹ������Ȼ��Ϊȼ�ϵĹܵ�ȼ����Ϊ��ֹȼ��й¶���Σ�գ����а�װ����ͼ��ʾ����������ش�
�ŷɼ�ʹ������Ȼ��Ϊȼ�ϵĹܵ�ȼ����Ϊ��ֹȼ��й¶���Σ�գ����а�װ����ͼ��ʾ����������ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
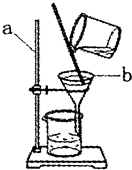
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��Ӧǰ | ��Ӧ�� |  | ||
| A | B | C | D | |
 |  |  |  | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
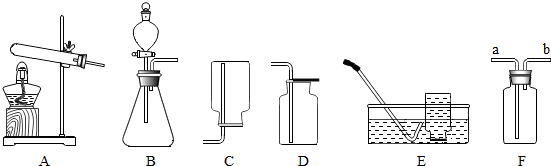
 ��ͼΪ����װ�ã�����ʵ��ش��������⣺
��ͼΪ����װ�ã�����ʵ��ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
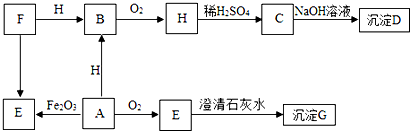
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ���� | �� | �� | �� | �� |
| ��Ӧǰ����/g | 20 | 3 | 2 | 20 |
| ��Ӧ������/g | x | 28 | 2 | 0 |
| A�� | �÷�Ӧ�Ļ�����ѧ��Ӧ����Ϊ���Ϸ�Ӧ | |
| B�� | ��Ӧ������ʵ�����ֵx=15 | |
| C�� | �˷�Ӧ�������ʺͶ����ʵ�������Ϊ5��4 | |
| D�� | ���ʱ�һ���Ƿ�Ӧ�Ĵ��� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com