

| ���� | ��֤���� | ʵ������ | ���� |
| ����1�� ����A�к�CaCO3 | ȡ��������A���Թ��У��μ�ϡ���ᣬ����Ϳ�г���ʯ��ˮ��С�ձ������Թܿڡ� | ��9�� | ����1���� |
| ����2�� ����A�к�BaCO3 | ȡ��������A���Թ��У��ȵ��� ��10�� ���ٵ��� ��11�� ��Һ�� | �����ݷų����ް�ɫ���� | ��12�� |
| ����3�� ����Ƶõ�NaCl�����л�����Na2SO4 | ȡ����NaCl�������Թ��У�����������ˮ�ܽ⣬�� ��13�� �� | ��14�� | ����3���� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | A | B | C | D |
| ʵ �� �� �� |  |  |  |  |
| ʵ�� Ŀ�� | ��֤CO2��H2O��Ӧ����H2CO3 | ̽����ȼ��ȼ�յ��������� | �ⶨ������O2�ĺ��� | ��֤�����غ㶨�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| A������ۡ������� | B������ۡ����������ʣ���������� |
| C����Ӧ�ų���������� | D����Ӧ�ų������������ܶ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
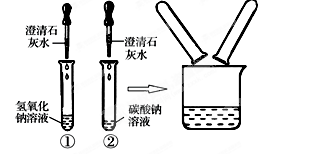
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ȡ��Ʒ���Թ��У� �� __. | _________��__________ | ��IJ�����ȷ |
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ȡ�����Թ��У����뼸��ϡ���� | û�����ݲ��� | �Լ��IJ��벻���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

| ʵ����� | ʵ����� |
| �ٶ�����̼����ȼ�գ�Ҳ��֧��ȼ�� | |
| �ڶ�����̼����ʹ��ɫʯ���ɫ | |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com