
����Ŀ����֪��25��ʱ��Ksp[Ni(OH)2]=2.0��10-15��Ksp[Fe(OH)3]=4.0��10-38������Fe2O3��Ag��Ni��ij�ͷϴ����������ᣬ���ˣ�����ΪAg��������Һ��c(Ni2+)=c(Fe3+)=0.4mol/L�������Һ�еμ�һ��Ũ�ȵ�NaOH��Һ��������Һ������䣩������˵������ȷ����
A.������ԣ�Ag��Ni
B.����NaOH��Һʱ���Ȳ���Ni(OH)2����
C.���ζ�����ҺpH=5ʱ����Һ��lg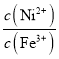 ԼΪ10
ԼΪ10
D.���ζ�����Һ������ʱ��Ni2+�ѳ�����ȫ
���𰸡�C
��������
A��Ag���������ᷴӦ����Ni�������ᷴӦ����˽�����ԣ�Ni��Ag����A����
B��c(Ni2+)=0.4mol/Lʱ��Ni2+�պÿ�ʼ����ʱ��Һ��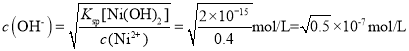 ��c(Fe3+)=0.4mol/Lʱ��Fe3+�պÿ�ʼ����ʱ��Һ��
��c(Fe3+)=0.4mol/Lʱ��Fe3+�պÿ�ʼ����ʱ��Һ�� �����Ȳ���Fe(OH)3��������B����
�����Ȳ���Fe(OH)3��������B����
C����ҺpH=5ʱc(H+)=10-5mol/L����Һ��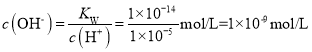 ��
��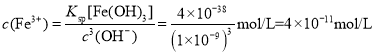 ��
��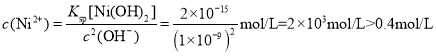 ����Ni2+�����c(Ni2+)=0.4mol/L����
����Ni2+δ������c(Ni2+)=0.4mol/L����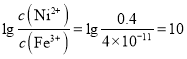 ����C��ȷ��
����C��ȷ��
D������Һ������ʱ��c(OH-)=1��10-7mol/L����ʱ��Һ��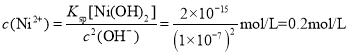 ����Ni2+δ������ȫ����D����
����Ni2+δ������ȫ����D����
�ʴ�Ϊ��C��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ�ﵽ����ʵ��Ŀ�ģ���Ӧ��ʵ�鷽���Լ���ؽ��;���ȷ���ǣ� ��
ѡ�� | ʵ��Ŀ�� | ʵ�鷽�� | ��ؽ��� |
A | ������ˮ��pH |
| pH��ֽ������ |
B | ̽��������(C5H12) ���ѽ� |
| C5H12�ѽ�Ϊ���ӽ�С��������ϩ�� |
C | ʵ���¶ȶ�ƽ���ƶ���Ӱ�� |
| 2NO2(g) |
D | ��AlCl3��Һ�Ʊ�AlCl3���� |
| AlCl3�е�����ܼ�ˮ |
A.AB.BC.CD.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ϳ�NH3�����H2����ú��H2O��Ӧ�Ƶã�������һ����ӦΪ��CO��g��+H2O��g��![]() CO2��g��+H2��g�� ��H��O�������COת���ʿɲ��õķ��������Тٽ����¶Ȣ�����ѹǿ��ʹ�ô���������CO��Ũ�Ȣ�����ˮ������Ũ�ȣ�������ȷ������ǣ� ��
CO2��g��+H2��g�� ��H��O�������COת���ʿɲ��õķ��������Тٽ����¶Ȣ�����ѹǿ��ʹ�ô���������CO��Ũ�Ȣ�����ˮ������Ũ�ȣ�������ȷ������ǣ� ��
A. �٢ڢ�B. �ܢ�C. �٢�D. ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������л������HCl ��NaOH ��NH4Cl ��CH3COONa ��CH3COOH ��NH3H2O��Na2CO3���������ĸ�����ʾ�����ʻش��������⡣
(1)����Һ������Ũ�ȴ�С˳��Ϊ______________________�������ӷ���ʽ��ʾ����Һ�Լ��Ե�ԭ��_______________________________��
(2)�����£�pH=11�Ģܵ���Һ�У���ˮ���������c(OH-)=____________����֪�����¢ݺ͢ĵ��볣����Ϊ1.7��10-5 mol��L-1����Ӧ��CH3COOH+NH3H2O![]() CH3COO-+NH4++H2O��ƽ�ⳣ��Ϊ______________��
CH3COO-+NH4++H2O��ƽ�ⳣ��Ϊ______________��
(3)�����£�����pHֵ��ͬ�Ģٺ͢�������Һ������˵������ȷ����________��
A.������Һ��ˮ�ĵ���̶���ͬ B.c(CH3COO-)=c(Cl-)
C.c(CH3COOH)>c(HCl) D.���Ũ�ȵ�����������Һ��Ӧ���������ĵ������
(4)�����£���0.10 mol/L�Ģ���Һ��0.30 mol/L������Һ�������ϣ���ַ�Ӧ��ָ������£���Һ��pH=________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����

A. ��ͼ��֪�������¶ȴ����Ƶ�ˮ��̶�����
B. ��ͼ�ҿ�֪��a��Kw����ֵ��b��Kw����ֵ��
C. ��ͼ����֪����ӦA(g)+B(g) ![]() 2C(g)�����ȷ�Ӧ
2C(g)�����ȷ�Ӧ
D. ��ͼ����֪����ӦC(���ʯ��s)= C(ʯī��s)���ʱ���H=��H1����H2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ҹ��涨������ˮ�����ŷŵ��������Ũ��Ϊ0.005mg/L���������ӷ�ˮ�ɲ��û�ѧ���������Իش��������⣺
(1)������[Cd3(PO4)2]�����ܽ�ƽ�ⳣ���ı���ʽKsp=___��
(2)һ���¶��£�CdCO3��Ksp=4.0��10-12��Cd(OH)2��Ksp=3.2��10-14����������ˮ�е��ܽ������ϴ����___��
(3)��ij���ӷ�ˮ�м���Na2S����S2-Ũ�ȴﵽ7.9��10-8mol/Lʱ��ˮ����Cd2+Ũ��Ϊ___mol/L��(��֪��Ksp(CdS)=7.9��1027)����ʱ�Ƿ����ˮԴ����______(����������������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±����и��������У�����֮��ͨ��һ����Ӧ����ʵ������ͼ��ʾת������
ѡ�� | X | Y | Z |
A | Na | NaOH | NaCl |
B | Si | SiO2 | Na2SiO3 |
C | Cl2 | HClO | NaClO |
D | NO | NO2 | HNO3 |

A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������л��ϳɡ���ص�����������Ҫ�����á�
I. ![]() ���Ʊ���Ӧ������ͼ��ʾ��
���Ʊ���Ӧ������ͼ��ʾ��

(1)�Ԫ����Ԫ�����ڱ��е�λ��_______________________��
(2)д��A�ĵ���ʽ___________________________��
(3)![]() ���л��ϳ��г��õĻ�ԭ������д����Ӧ�۵Ļ�ѧ����ʽ_________________��
���л��ϳ��г��õĻ�ԭ������д����Ӧ�۵Ļ�ѧ����ʽ_________________��
II.�������������������ӵ�ص���ѡ�缫���ϣ���������Ϊ������ʯīΪ����������������李��Ȼ�﮻����Һ��������������﮳�������800�����ҡ����������Χ�������Ƶá�������ӵ���У���Ҫһ���л��ۺ�����Ϊ������֮�������Ǩ�ƵĽ��ʣ����л��ۺ���ĵ���֮һ(��M��ʾ)�Ľṹ��ʽ���£�

��ش��������⣺
(4)�Ʊ���������ﮱ����ڶ��������Χ�н��У���ԭ����_______________��
(5)����������������﮵ĵ缫��ӦʽΪ___________________��
(6)д��M����������������Һ��Ӧ�Ļ�ѧ����ʽ_____________________��
(7)�õ�س��ʱ�����������������������������ŵ�ʱ�����ĵ缫��ӦʽΪ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��PVAc��һ�־��������Ե���֬���ɺϳ���Ҫ�߷��Ӳ���M���ϳ�·�����£�
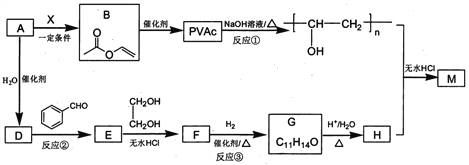
��֪��R��R�@��R�@�@ΪHԭ�ӻ�����
I. R'CHO+ R"CH2CHO![]()
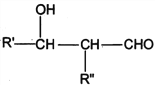
![]()
![]()
II. RCHO+![]()
![]()
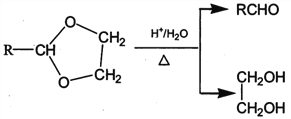
��1����״���£�4.48L��̬��A��������5.2g����A�Ľṹ��ʽΪ___________________��
��2����֪A��BΪ�ӳɷ�Ӧ����X�Ľṹ��ʽΪ_______��B�й����ŵ�������_________��
��3����Ӧ�ٵĻ�ѧ����ʽΪ______________________��
��4��E��ʹ������Ȼ�̼��Һ��ɫ����Ӧ�ڵķ�Ӧ�Լ���������_______________________��
��5����Ӧ�۵Ļ�ѧ����ʽΪ____________________________��
��6����E��F��G��H��ת�������У��Ҷ�����������__________________________��
��7����֪M�������г������⣬��������Ԫ��״�ṹ����M�Ľṹ��ʽΪ_________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com