
����Ŀ�������仯�������ճ���������;�ȽϹ㷺��
��1����������(Na2FeO4)��һ�����͵���ɫ��������������ز��ϡ���Fe(NO3)3��NaClO��Ϻ��ڼ��������·�����Ӧ���Ƶø������ƣ��÷�Ӧ�����ӷ���ʽΪ_____________________________________��
��2������������(Fe3O4)���������ϡ���������ϡ������͵��Ӳ��ϵȡ�����������Ŀǰ�Ʊ�����Fe3O4����Ҫ����֮һ����������ͼ��ʾ��
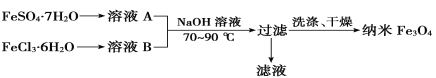
��Ϊ�õ��ϴ���������Fe3O4��FeSO4��7H2O��FeCl3��6H2O�����ʵ���֮�����Ϊ________����ʵ�ʲ���ʱ��ȴ���ѿ�����һ������ԭ����___________________________________________��
�������Ͷ�ϱ������£�����Fe3O4�Ƿ������ȫ��ʵ�������_________________________��
(3)�̷�(FeSO4��7H2O)������ȱ����ƶѪҩƷ����Ҫ�ɷ֡��ⶨ�̷���Ʒ��FeSO4��7H2O�����ķ������£�
a����ȡ3.0 g�̷���Ʒ�����Ƴ�250.00 mL��Һ��
b����ȡ25.00 mL a����Һ����ƿ�У�
c����0.01000 mol��L��1����KMnO4��Һ�ζ����յ㣬����KMnO4��Һ��ƽ�����Ϊ20.00 mL���ζ�ʱ������Ӧ�����ӷ���ʽΪ5Fe2����MnO4����8H��===5Fe3����Mn2����4H2O��
���ζ��յ��������_______________________________________________________________________��
����Ʒ��FeSO4��7H2O����������Ϊ________(С�������1λ����)��
���������������ⶨ����Ʒ��FeSO4��7H2O����������ƫ��(�ⶨ�����в��������ɺ���)�����ܵ�ԭ����________________________________________________________________________��
���𰸡�2Fe3����3ClO����10OH��===2FeO42����3Cl����5H2O 1��2 Fe2�����ױ�����ΪFe3�� ȡ�����ϲ���Һ���μ�KSCN��Һ������Һ�����ɫ������������ȫ ��Һ���dz��ɫ�Ұ�����ڲ���ɫ 92.7% ��Ʒ�д������������ʻ���Ʒ���ֱ�����
��������
��1��������֪����Ӧ��ΪFe3����ClO����OH������������FeO42����ClO������ԭӦ����Cl�������ݵ�ʧ�����غ㡢����غ��ԭ���غ����ƽ�����ӷ���ʽ2Fe3����3ClO����10OH��=2FeO42����3Cl����5H2O��
��2����Fe3O4��Fe2����Fe3���ĸ�����Ϊ1��2����ԭ����FeSO4��7H2O��FeCl3��6H2O�����ʵ���֮��Ϊ1��2��Fe2���н�ǿ�Ļ�ԭ�ԣ��ڿ������ױ�������Ӱ��Ͷ�ϱȣ�����ʵ�ʲ���ʱ�����ѿ�����һ������
�����Ͷ�ϱ�ʱ��������ȫ������Һ�в�����Fe2����Fe3�����ʿ���KSCN��Һ���飬��ȡ�����ϲ���Һ���μ�KSCN��Һ������Һ�����ɫ������������ȫ��
��3���ٵζ����յ�ʱ����������һ��KMnO4��Һ���ٷ�Ӧ��ʹ��Һ��dz��ɫ�����Եζ��յ����������Һ���dz��ɫ�Ұ�����ڲ���ɫ��
���ɷ�Ӧ�����ӷ���ʽ��֪��n(Fe2��)��5n(MnO4-)��5��0.01000mol/L��0.0200L��![]() ��0.01mol���� w��FeSO4��7H2O����
��0.01mol���� w��FeSO4��7H2O����![]() ��100����92.7%��
��100����92.7%��
������������ƫ�ͣ�˵����Һ�������ʻ�Fe2���ѱ��������������ı��MҺ������١�


 ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijѧϰС����ʵ�����о�SO2��Ba��NO3��2��Һ�ķ�Ӧ��
ʵ�飺��ʢ��2mL 0.1mol/L Ba��NO3��2��Һ���Թ��У�����ͨ��SO2���壬�Թ����а�ɫ����������Һ���Ϸ�����dz��ɫ��
̽��1����ɫ����������ԭ��
��1����ɫ������_________���ѧʽ����
��2��������ɫ����������ԭ��ͬѧ��Ϊ��NO3��������SO2����ͬѧ��Ϊ����Һ���ܽ��O2������SO2��
��֧�ּ�ͬѧ�۵��ʵ��֤����______________��
����ͬѧͨ������ʵ��֤�����Լ����Ʋ���ȷ�������ʵ�鷽����
ʵ����� | ʵ������ |
��2mL___mol��L ___��Һ���ѧʽ����ͨ��SO2 | ____________ |
̽��2��������SO2�Ĺ����У�NO3����O2������������Ҫ���á�
ʵ����� | ʵ������ |
���ձ��м�������˵�0.1 mol��L��BaCl2��Һ25mL���ټ���25mLֲ���ͣ���ȴ�����£���pH�������ⶨ��ҺpH��ʱ�䣨t���ı仯���� |
ͼ1����BaCl2 ����������Һ��ͨ��SO2 |
���ձ��зֱ����25mL 0.1 mol��L��BaCl2��Һ��Ba��NO3��2��Һ��ͨ��SO2����pH�������ֱ�ⶨ��ҺpH��ʱ�䣨t���仯�����ߡ� |
ͼ2���ֱ���BaCl2��Ba��NO3��2��Һ��ͨ��SO2 |
��3��ͼ1�������������£���BaCl2��Һ�г���ͨ��SO2����ҺpH�½�����Ϊ_________���÷���ʽ��ʾ����
��4��ͼ2��BaCl2��Һ�з�����Ӧ�����ӷ���ʽΪ___________��
��5����������ͼ����ó��Ľ�����_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��̼���������CH3OCOOCH3�����DMC����һ��Ӧ��ǰ���㷺���²��������ܱ������а�n(CH3OH)��n(CO2)=2��1Ͷ��ֱ�Ӻϳ�DMC����Ӧ����ʽΪ2CH3OH(g)+CO2(g)![]() CH3OCOOCH3(g)+H2O(g)��һ�������£�ƽ��ʱCO2��ת������ͼ��ʾ������˵������ȷ����
CH3OCOOCH3(g)+H2O(g)��һ�������£�ƽ��ʱCO2��ת������ͼ��ʾ������˵������ȷ����

A. �÷�Ӧ������ӦΪ���ȷ�Ӧ
B. ѹǿp2��p1
C. X���Ӧ��ƽ�ⳣ��Ϊ0.5
D. X��Y��Z�����Ӧ�ij�ʼ��Ӧ���ʵĹ�ϵΪZ>X>Y
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����9gͭ�����Ļ����Ͷ��100mlϡ�����У���ַ�Ӧ��õ���״����1.12LNO��ʣ��4.8g��������������100ml��Ũ�ȵ�ϡ���ᣬ������ȫ�ܽ⣬�ֵõ���״����1.12LNO������Ӧ�����Һ�м���KSCN��Һ����Һ����죬������˵����ȷ����
A. ԭ�������ͭ������0.065mol
B. ϡ��������ʵ���Ũ��Ϊ4.0mol��L��1
C. ��һ��ʣ���4.8g����Ϊͭ����
D. ��Ӧ�����Һ���ټ����ϡ����100ml���ֵõ���NO�ڱ�״���µ����Ϊ0.56L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ�˲ⶨ�Ҵ��Ľṹʽ���������������ˮ�ƾ����Ʒ�Ӧ��ʵ��װ�úͲⶨ���������װ�ý���ʵ�顣�ɹ�ѡ�õ�ʵ��������ͼ��ʾ��
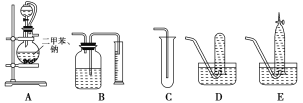
��ش��������⣺
(1)���������������ȷװ����________(��д���)��
(2)װ����A���ֵķ�Һ©����������ƿ֮�����ӵĵ��������������________(��д���)��
A����ֹ��ˮ�ƾ��ӷ�
B����֤ʵ��װ�ò�©��
C��ʹ��ˮ�ƾ�������
(3)ʵ��ǰԤ�Ƚ�С�����ڶ��ױ����ۻ���С���飬��ȴ������ƿ�У���Ŀ����
________________________________________________________________________��
(4)��֪��ˮ�ƾ����ܶ�Ϊ0.789 g��cm��3����ȡ2.0 mL�ƾ�����Ӧ��ȫ��(�ƹ���)���ռ�390 mL���塣���Ҵ��������ܱ���ȡ��������ԭ����Ϊ________���ɴ˿�ȷ���Ҵ��ĽṹʽΪ________________������____________________________________________________��
(5)ʵ�����ⶨ�Ľ��ƫ�ߣ����������ԭ����________(��д���)��
A����ʵ���������½���
B����ˮ�ƾ��л������״�
C����ˮ�ƾ����Ƶķ�Ӧ������ȫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ϳɰ�������ʾ��ͼ���£�
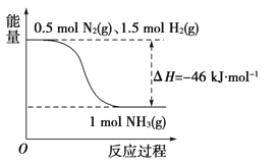
��1����֪�ֱ��ƻ�1 mol N![]() N����1 mol H-H��ʱ��Ҫ���յ�����Ϊ946 kJ��436 kJ�����ƻ�1 mol N-H����Ҫ���յ�����Ϊ________ kJ��
N����1 mol H-H��ʱ��Ҫ���յ�����Ϊ946 kJ��436 kJ�����ƻ�1 mol N-H����Ҫ���յ�����Ϊ________ kJ��
��2��N2H4����ΪNH3�����е�H����NH2ȡ���IJ����������ʱ��N2H4(g)Ϊȼ�ϡ�NO2Ϊ�����������߷�Ӧ����N2��H2O(g)��
��֪��N2(g)��2O2(g)===2NO2(g)��H1����67.7 kJ��mol��1
N2H4(g)��O2(g)===N2(g)��2H2O(g)��H2����534 kJ��mol��1
��1 mol N2H4��NO2��ȫ��Ӧ���Ȼ�ѧ����ʽΪ____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������Ԫ��(����Ԫ����ȥ)�����ڱ��е�λ������:

(1) A��Ԫ�ط�����________��
(2) D�����ڱ��е�λ����_________________________________��
(3) E��G��T��ԭ�Ӱ뾶�ɴ�С��˳����_________(��Ԫ�ط���)��
(4)��Ԫ��(34Se)��Dͬ���壬��ǽ����Ա�D_________(����ǿ����������)����ԭ�ӽṹ�ǶȽ�����ԭ��:___________________________________________________________________��
(5) RԪ�ص�ԭ�ӽṹʾ��ͼΪ_________________��
(6) E��G��J����Ԫ������������Ӧˮ��������֮���ܷ�Ӧ�����ӷ���ʽ�ֱ�Ϊ:H+ +OH-��H2O��_________________________________��______________________��
(7) E��D��Ԫ�����γ�ԭ�Ӹ�����2:1��1:1�����ֻ����2:1 �ͻ�����ĵ���ʽΪ______________________________��1:1�ͻ�����Ļ�ѧʽ�Լ�������ѧ��������_____________________________��
(8)A��M�γɵķ��ӿ�����______________(����ĸ���)��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������л�ѧ����:
(1)CH4��Cl2��Ӧ����һ�ȼ���Ļ�ѧ����ʽ: _________����Ӧ������_________��
(2)��ϩʹBr2/CCl4��Һ��ɫ��������Ӧ����ʽ_________����Ӧ������_________��
(3)�Ҵ���������Ӧ������ȩ�Ļ�ѧ����ʽ_________����Ӧ������_________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com