
����Ŀ����ҵ���Ѿ�ʵ��CO2��H2��Ӧ�ϳɼ״�����һ���¡������ܱ������г���2molCO2��6molH2��һ�������·�����Ӧ��CO2(g)+3H2(g)=CH3OH(g)+H2O(g)�����CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ����ش�

��1�����ܱ��������ݻ���___��
��2���ﵽδƽ��״̬��ʱ����___min(�3������10��)��
��3����ǰ3min�ڣ���H2Ũ�ȵı仯��ʾ�ķ�Ӧ����v(H2)=___mol/(L��min)��
��4��10minʱ��ϵ��ѹǿ�뿪ʼʱѹǿ֮��Ϊ__��
��5����ƽ���H2O(g)�����ʵ���������___��
��6����֪����CO(g)+2H2(g)=CH3OH(g) ��H=-90.1kJ/mol����CO(g)+H2O(g)=CO2(g)+H2(g����H=-41.1kJ/mol����CO2��H2��Ӧ�ϳ�CH3OH(g)���Ȼ�ѧ����ʽ__����Ӧ��10min�����ų�������Ϊ__��
���𰸡�2L 3 0.225 mol/(L��min) ![]() 30% CO2(g)+3H2(g)=CH3OH(g)+H2O(g) ��H =-49 kJ/mol 98 kJ
30% CO2(g)+3H2(g)=CH3OH(g)+H2O(g) ��H =-49 kJ/mol 98 kJ
��������
��һ���¡������ܱ������г���2molCO2��6molH2����ͼ���֪��CO2�ij�ʼŨ��Ϊ1mol/L�������= ![]() =2L���������ʵ�Ũ�Ȳ�����ʱ��ı仯���仯������Ӧ����ƽ��״̬����ͼ���֪��3min��CO2��CH3OH��Ũ�Ȼ��ڷ����仯��10min��CO2��CH3OH��Ũ�Ȳ������仯����3min��δƽ��״̬��10minΪƽ��״̬������v=
=2L���������ʵ�Ũ�Ȳ�����ʱ��ı仯���仯������Ӧ����ƽ��״̬����ͼ���֪��3min��CO2��CH3OH��Ũ�Ȼ��ڷ����仯��10min��CO2��CH3OH��Ũ�Ȳ������仯����3min��δƽ��״̬��10minΪƽ��״̬������v=![]() ���㷴Ӧ���ʣ���ͼ��֪���ڵ�3minʱ������v=
���㷴Ӧ���ʣ���ͼ��֪���ڵ�3minʱ������v=![]() ��֪������̼��Ӧ���ʣ�������̼������������֮�ȵ������ǵļ�����֮�ȣ�����������ķ�Ӧ���ʣ�
��֪������̼��Ӧ���ʣ�������̼������������֮�ȵ������ǵļ�����֮�ȣ�����������ķ�Ӧ���ʣ�
��4��10minʱ�ﵽƽ�⣬��������ʽ�����10min����������ʵ����뿪ʼʱ�����ʵ���֮�ȣ�����ͬ����£������ѹǿ֮�ȵ������ʵ���֮�ȣ��ɼ����10minʱ��ϵ��ѹǿ�뿪ʼʱѹǿ֮��Ϊ����ƽ���H2O(g)�����ʵ���������=nH2O/n�ܣ����ø�˹���ɢ�-�ڿɵõ���ȷ���Ȼ�ѧ����ʽ��
��1����һ���¡������ܱ������г���2molCO2��6molH2����ͼ���֪��CO2�ij�ʼŨ��Ϊ1mol/L�������=n/v=2/1=2L��
�ʴ�Ϊ��2L��
��2���������ʵ�Ũ�Ȳ�����ʱ��ı仯���仯������Ӧ����ƽ��״̬����ͼ���֪��3min��CO2��CH3OH��Ũ�Ȼ��ڷ����仯��10min��CO2��CH3OH��Ũ�Ȳ������仯����3min��δƽ��״̬��10minΪƽ��״̬��
�ʴ�Ϊ��3��
��3����ͼ��֪���ڵ�3minʱ������v=![]() ��֪������̼��Ӧ���ʣ�������̼������������֮�ȵ������ǵļ�����֮�ȣ�����������ķ�Ӧ���ʣ�����ͼ���֪��vCO2=
��֪������̼��Ӧ���ʣ�������̼������������֮�ȵ������ǵļ�����֮�ȣ�����������ķ�Ӧ���ʣ�����ͼ���֪��vCO2=![]() =
=![]() =0.075 mol/(L��min)�����ݷ�ӦCO2(g)+3H2(g)=CH3OH(g)+H2O(g)��������̼������������֮�ȵ���1��3����vCO2��vH2=1��3��vH2==3vCO2=3��0.075 mol/(L��min)=0.225 mol/(L��min)��
=0.075 mol/(L��min)�����ݷ�ӦCO2(g)+3H2(g)=CH3OH(g)+H2O(g)��������̼������������֮�ȵ���1��3����vCO2��vH2=1��3��vH2==3vCO2=3��0.075 mol/(L��min)=0.225 mol/(L��min)��
�ʴ�Ϊ��0.225 mol/(L��min)��
��4���г�����ʽ CO2(g)+3H2(g)=CH3OH(g)+H2O(g)
ʼ mol/L 1 3 0 0
�� mol/L 0.75 2.25 0.75 0.75
ƽ mol/L 0.25 0.75 0.75 0.75
10min��ﵽƽ�⣬10minʱ��ϵ��ѹǿ�뿪ʼʱѹǿ֮��Ϊ![]() =
=![]() ��
��
�ʴ�Ϊ��![]() ��
��
��5����ƽ���H2O(g)�����ʵ�������=![]() =
=![]() =30%��
=30%��
�ʴ�Ϊ��30%��
��6����֪����CO(g)+2H2(g)=CH3OH(g) ��H1=-90.1kJ/mol����CO(g)+H2O(g)=CO2(g)+H2(g����H2=-41.1kJ/mol�����ø�˹�ɢ�-�ڵõ�CO2(g)+3H2(g)=CH3OH(g)+H2O(g) ��H=��H1-����H2��=-90.1+41.1=-49 kJ/mol��1molCO2��ȫ��Ӧ���ɷų�49 kJ������������Ŀ������2molCO2��ȫ��Ӧ����ų�������98 kJ��������
�ʴ�Ϊ��CO2(g)+3H2(g)=CH3OH(g)+H2O(g) ��H =-49 kJ/mol��98 kJ��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����A��B��C��D���ֶ�����Ԫ�أ����ǵ�ԭ��������A��D����������֪A��Bԭ������ͬ�ĵ��Ӳ�������A��L���������K���������������Cȼ��ʱ���ֻ�ɫ���棬C�ĵ����ڵ�ȼ��������B�ĵ��ʳ�ַ�Ӧ�����Եõ���D�ĵ�����ɫ��ͬ�ĵ���ɫ��̬������Ը������������ش�
(1)д��AB2�ĵ���ʽ��______________��
(2)�õ���ʽ��ʾC2B���γɹ��̣�__________________________________��
(3)д������ɫ��̬��������ˮ��Ӧ�Ļ�ѧ����ʽ��_______________________________��
(4)����Ƽ�ʵ�飬�Ƚ�Ԫ��B��D�Ľ�����(��ǽ�����)ǿ����________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ͼ���ѧ�о�����TiO2-x�����д��ڵľ���ȱ����������ߵ����Ժ��ṩþ���Ӵ�λ������������þ��ظ������ϣ��õ�صĹ���ԭ����ͼ��ʾ������˵����ȷ���ǣ� ��
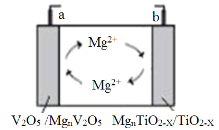
A.�ŵ�ʱ��aΪ��ظ���
B.���ʱ��Mg2+��b�缫�ƶ�
C.�ŵ�ʱ��a���ĵ缫ʽΪnMg2++V2O5+2ne-=MgnV2O5
D.���ʱ��b���ĵ缫ʽΪMgnTiO2-x-2ne-=nMg2++TiO2-x
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2019����ʷ�������֯��ȫ��ƻ���12��4�շ������棺�о���ʾ��ȫ�������̼�ŷ�������������CO2���ۺ������ǽ�������������Ч;����
(1)CO2�������Ƽ״����йط�Ӧ�����ڲ�ͬ�¶��µĻ�ѧƽ�ⳣ�����±���ʾ��
��ѧ��Ӧ | ƽ�ⳣ�� | ||
500�� | 700�� | 800�� | |
��.H2(g)+CO2(g) H2O(g)+CO(g) | 1.0 | 1.70 | 2.52 |
��.2H2(g)+CO(g) CH3OH(g) | 2.5 | 0.34 | 0.15 |
��.3H2(g)+CO2(g) CH3OH(g)+H2O(g) ��H | |||
����H___0(������������������������)��
����֪��Ӧ�������ʷ���ʽ������=k����c3(H2)��c(CO2)������=k����c(CH3OH)��c(H2O)��k����k��Ϊ���ʳ�������Ӧ�ﵽƽ��������¶ȣ�k������ı���___k������ı���(��������������С��������������)��
��500��ʱ������ݵ��ܱ������м���1molCO2��1molH2�����Ʒ�Ӧ����ֻ������Ӧ�����ﵽƽ���ֻ�ı�������������ʹCO��ƽ����������������___(��ѡ����ĸ)��
A.����ѹǿ B.�����¶� C.��ͨ������ʵ���CO2��H2 D.���������ˮ
(2)��200��ʱ����5L����ѹ�Ƶĺ����ܱ�������ͨ��2molCO2��2molCH4������ӦCH4(g)+CO2(g)2H2(g)+2CO(g)����ó�ʼѹǿΪP0kPa����Ӧ��������������ѹǿ(P)��ʱ��(t)�仯(��Ӧ�ﵽƽ��ʱ���¶�����ʼ�¶���ͬ)��ͼ��ʾ��
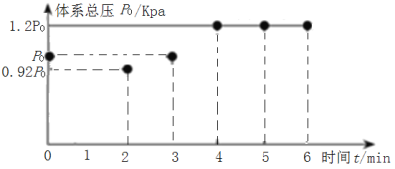
�ٸ÷�Ӧ�����д�0min��2minѹǿ�仯ԭ����___��
��0��4min�ڣ���Ӧ��ƽ����Ӧ������(CO2)=___��
����ƽ���ѹ����ƽ��Ũ�ȱ�ʾ�Ļ�ѧƽ�ⳣ��Kp=___��[�����ѹ(p��)=������ѹ(p��)�������������]
(3)��ѧ���������CO2��CH4�Ʊ����ϳ�����(CO��H2)���ܵķ�Ӧ������ͼ��ʾ��
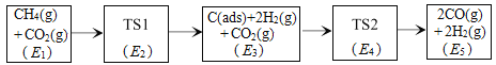
ע��C(ads)Ϊ�����Ի���̿�������ڰ��������༰��Ŀ���������������[���һ��������1��CH4(g)+1��CO2(g)�����������ΪE1eV����λ��eV]�����У�TS��ʾ����̬��
��CH4(g)+CO2(g) 2H2(g)+2CO(g)��H=___kJ��mol-1(��֪��1eV=1.6��10-22kJ)
����E4+E1��E3+E2��������Ʊ����ϳ�������Ӧ���ʵķ�Ӧ����ʽΪ___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ���������������������������Ļ�Ϸ�ĩ�У�����100ml2mol/L�����ᣬǡ��ʹ�������ȫ�ܽ⣬���ų�448ml�����壨SPT������������Һ�м���KSCN��Һ��Ѫ��ɫ���֣�������ͬ����������������ĩ������ͬ������һ����̼��Ӧ���ɵõ����������ǣ� ��
A.������B.2.8gC.5.6gD.11.2g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������װ��������ʵ�飬�������ǣ� ����

A. ͼ�٣���֤H2CO3����ǿ��H2SiO3 B. ͼ�ڣ��ռ�CO2��NH3
C. ͼ�ۣ�����Na2CO3��Һ��CH3COOC2H5 D. ͼ�ܣ�����C2H5OH��CH3COOC2H5
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ��ȤС�����Fe2+��Fe3+ת��ʵ�飺��FeCl2��Һ�м�������KSCN��Һ����Һ�����Ա仯���ټ�������˫��ˮ����Һ��죬�����μ���������������Һ��ɫ��ȥ��ͬʱ���������ɣ�������Դ��쳣����չ��̽������ش��й����⣺
��1����С����ڲ��������ԭ�������ֲ²⣺
�²�һ��__________________________________________��
�²������ɫ��ȥ������SCN-��H2O2������ͬʱ�����������п��ܺ��е�����������̼����������
��2����ѧС����Բ²����������ʵ������֤����ɷ֣�
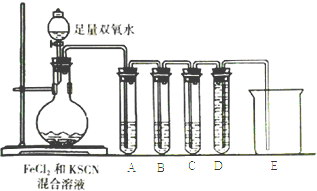
���Թ�A��ʢ��Ʒ����Һ����ʵ����Ʒ����Һ��ɫ��֤�������к���____________��
���Թ�B�е���Һ�����Ը��������Һ����Ŀ����________________��Ԥ���Թ�B�е�������________________________________��
���Թ�C��ʢ�г���ʯ��ˮ��Ŀ����___________________���Թ�D���ձ���������______________��������ʵ��֤��SCN-�ܱ�H2O2��������д���÷�Ӧ�����ӷ���ʽ��___________________________________��
��3��������Ŀ��Ϣ������ʵ���ƶϣ�Fe2+��SCN-�л�ԭ�Խ�ǿ����_______��������_________��
��4��������ΪSCN-������������ܻ�����������ӣ������һ����ʵ��֤���ü����Ƿ���ȷ_____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������һ����ɫ��̬ɱ�������ṹ��ʽΪ �������������������һ�������·�Ӧ�Ƶá�����˵������ȷ���ǣ� ��
�������������������һ�������·�Ӧ�Ƶá�����˵������ȷ���ǣ� ��
A.��������ĵ���ʽ![]()
B.��������ˮ��CH3COOH=CH3COO-+H+
C.���������к��м��Թ��ۼ��ͷǼ��Թ��ۼ�
D.�Ʊ���������Ļ�ѧ��Ӧ����ʽ��CH3COOH+H2O2![]() CH3COOOH+H2O
CH3COOOH+H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����20mL0.5mol��L-1�Ĵ�����Һ����μ���һ�����ʵ���Ũ�ȵ��ռ���Һ�������Һ���¶�������NaOH��Һ�������ϵ��ͼ��ʾ�������й�˵����ȷ����( )
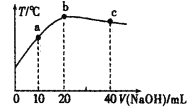
A.a�㣺c(CH3COOH)��c(OH-)+c(Na+)
B.����ĵ��볣����b�㣼a��
C.b�㣺c(CH3COOH)+c(CH3COO-)=0.5mol��L-1
D.c�㣺n(OH-)-n(H+)-n(CH3COOH)=0.01mol
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com