
ij�������������Al��(NH4)2SO4��MgCl2��AlCl3��CuCl2�е�һ�ֻ�����ɣ�
�ֶԸû����������ʵ�飬����������й�������ͼ��ʾ��������������ѻ���ɱ�״���µ��������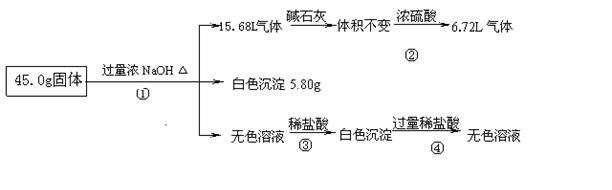
�ش��������⣺
�Ż�������Ƿ����CuCl2 ����ǡ�����
�ƻ�������Ƿ����(NH4)2SO4 ����ǡ���������ж������� ��
��д����Ӧ���е����ӷ���ʽ
��
(4)����ݼ������жϻ�������Ƿ���AlCl3��˵����ļ������ݣ���Ҫ��д������̣���
___________________________________________________________________________��
�ŷ�1�֣� ���ǣ�1�֣� ����ͨ��Ũ�������8.96L��(ֻҪ���������ټ��ɵ÷�) ��1�֣�
��3��H++OH-=H2O H2O+AlO2-+H+=Al(OH)3�� ����2�֣�
��4����������Ϣ���Ƶ�һ������Al��(NH4)2SO4��MgCl2�������ʣ���������֮�͵���41.3g��С�ڹ���������45.0g������ԭ�������һ������AlCl3����3�֣�
����������������ݷ�Ӧ����ͻ�ѧ�����ж����ʵ���ɣ����ݼ�������������Һ�õ���ɫ���������Ƴ����Ȼ�ͭ�����ɵ�����ͨ����ʯ��������䣬��ͨ��Ũ������٣�˵�������к��а�����������к�������泥��ۼ������ᣬ�Ⱥ͢��й������������Ʒ�Ӧ��Ȼ����������ƫ�������Ӧ�����ɰ�ɫ����������������ɫ����5.80gΪ������þ����������������Ȼ�þ������������ͨ��Ũ�������8.96L��Ϊ��������������������淋�������ʣ���6.72L����Ϊ��������������Һ��Ӧ���ɵ�������Ȼ�����Al��(NH4)2SO4��MgCl2��������֮��41.3g��С�ڹ���������45.0g������ԭ�������һ������AlCl3��
���㣺���⿼�����ʵ��ƶϡ����ӷ���ʽ����д����ѧ���㡣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����������ʣ���Al����ϡ�����NaHCO3����Һ�����������������Һ������ˮ�Ҵ���������NaOH����NaHSO4����CO2���ش��������⣨����Ӧ���ʵ������д��
��1�����Ե������ ��
��2�����ڷǵ���ʵ��� ��
��3����д���۵ĵ��뷽��ʽ ��
��4��д�����е����ʵ���Һ������Һ��Ӧ�����ӷ���ʽ ��
��5��д��������е����ʵ���Һ��Ӧ�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ֱ���һ���Լ������������л�����������ʳ�ȥ��������Ϊ��������ʣ�
| ���� | �������Լ� | �й����ӷ���ʽ |
| ��1��HNO3(H2SO4) | | |
| ��2��Cu(Fe) | | |
| ��3��NaCl(Na2CO3) | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��Һ����K+��Cu2+��Fe3+��Al3+��Fe2+��Cl����CO2��3��OH����NO��3��SO2��4�еļ��֣���֪����Һ�и��������ʵ���Ũ�Ⱦ�Ϊ0��2mol/L��������ˮ�ĵ��뼰���ӵ�ˮ�⣩��Ϊȷ������Һ�к��е����ӣ��ֽ��������µIJ�����
I��ȡ������Һ������KSCN��Һʱ�����Ա仯��
��ȡԭ��Һ����BaCl2��Һ���а�ɫ�������ɣ�
����ȡ��Һ�������ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ����ʱ��Һ��Ȼ���壬����Һ������������û�����ӡ�
���ƶϣ�
��l�����ɲ���I������ɵó��Ľ�����____��
��2�����ɲ���������ɵó��Ľ�����____��
��3���ɲ���������ɵó���Һ��һ�����е���������____��д���ӷ��ţ����������з�����Ӧ�Ļ�ѧ����ʽΪ____��
��4��������������������֪������Ϊԭ��Һ���������������____�֡�
��5����ȡl00mLԭ��Һ������������NaOH��Һ����ַ�Ӧ����ˣ�ϴ�ӣ����������أ��õ��Ĺ�������Ϊ g��д���˹������漰������ԭ��Ӧ�Ļ�ѧ����ʽ____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������������������й㷺Ӧ�á�
��ҵ�����ؾ�ʯ����Ҫ�ɷ�BaSO4��Ϊԭ���Ʊ�BaCl2���乤������ʾ��ͼ���£�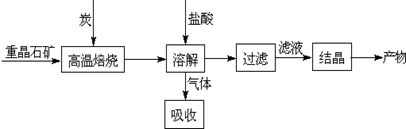
ij�о�С��������ϵã�
BaSO4��s��+4C��s�� 4CO��g��+BaS��s����H1=+571.2kJ?mol-1 ��
4CO��g��+BaS��s����H1=+571.2kJ?mol-1 ��
BaSO4��s��+2C��s�� 2CO2��g��+BaS��s����H2=+226.2kJ?mol-1 ��
2CO2��g��+BaS��s����H2=+226.2kJ?mol-1 ��
��1���ù���NaOH��Һ�������壬�õ����ơ��÷�Ӧ�����ӷ���ʽ�� ��
��2����ӦC��s��+CO2��g�� 2CO��g���ġ�H= ��
2CO��g���ġ�H= ��
��3��ʵ�������б�����������̿��ͬʱ��Ҫͨ���������Ŀ��������
�ٴ�ԭ�ϽǶȿ��� ��
�ڴ������Ƕȿ����٢�Ϊ���ȷ�Ӧ��̿��������Ӧ����ά�ַ�Ӧ������¡�
��4����С��ͬѧ���BaSO4��ˮ�еij����ܽ�ƽ������һ���о��������Ϸ�����ij�¶�ʱBaSO4��ˮ�еij����ܽ�ƽ��������ͼ��ʾ��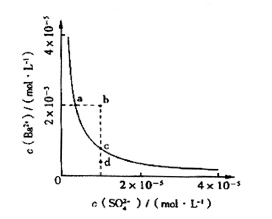
��С��ͬѧ����������ֹ۵㣺
�ٵ�����SO42-����Һ�м���Ba2+ ʹSO42-������ȫ�����ʱSO42-����Һ�е�Ũ��Ϊ0
�ڼ���Na2SO4����ʹ��Һ��a��䵽b��
��ͨ����������ʹ��Һ��d��䵽c��
��d����BaSO4��������
������ȷ���� ������ţ���
��ijȼ�ϵ����CaHSO4����Ϊ����ʴ���H+��������ṹ��ͼ��ʾ������ܷ�Ӧ�ɱ�ʾΪ2H2+O2�T2H2O��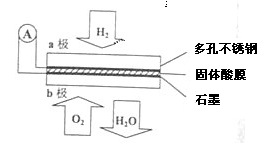
��ش�
��5��H+�� ��ͨ�����������ʴ��ݵ���һ������a����b����
��6��b���Ϸ����ĵ缫��Ӧ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij������ˮ��Һ�����ܺ������������е������֣�K+��Al3+��Fe3+��Mg2+��Ba2+��NH4+��Cl-��CO32-��SO42-���ֱַ�ȡ100mL�����ȷ���Һ��������ʵ�飺
�ٵ�һ�ݼӹ���NaOH��Һ����ȣ��ռ���0.02mol���壬�������ɣ�ͬʱ�õ���Һ�ס�
�������Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����պõ�1.02g���塣
�۵ڶ��ݼ�����BaCl2��Һ�����ɰ�ɫ��������������������ϴ�ӡ�����õ�11.65g���塣
����ʵ��ش��������⣺
��1���ɢٿ�֪���ڵ�����Ϊ ��Ũ���� mol��L-1��
�ɢڿ�֪���ڵ�����Ϊ ��Ũ���� mol��L-1��
�ɢۿ�֪���ڵ�����Ϊ ��Ũ���� mol��L-1��
��2������Һ��һ�������ڵ������� �������ӷ��ţ���
��3��ijͬѧͨ��������Ϊ����Һ��һ������K+������������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ij��ȤС��ģ���ȼҵ����ԭ����̽�����Ʒ�����ʡ���֪�����أ�
���������������������Һ��Ӧ����NaC1O���������ȵ�����������Һ��Ӧ������NaC1O��NaC1O3���������Խ���ʱKIֻ�ܱ�NaC1O�����������Խ�ǿʱ���ܱ�NaC1O3������
��1����С��������ͼ��ʾװ����ȡ��������Һ����Ҫ�ɷ�ΪNaClO������aΪ �������������������������NaClO�����ӷ���ʽΪ ��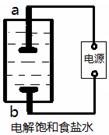
��2����С�齫0.784 L����״����Cl2ͨ��50.00 mL�ȵ�NaOH��Һ�У�����ǡ����ȫ��Ӧ��ϡ�͵�250.0 mL��
��ȡϡ�ͺ����Һ25.00 mL�ô����ữ���������KI��Һ����0.2000 mol��L��1 Na2S2O3��Һ�ζ���I2��2S2O32����2I����S4O62��������Na2S2O3��Һ10.00 mLʱǡ�õ����յ㡣
�ڽ������ζ������Һ�������ữ��ǿ���ԣ���������Na2S2O3��Һ�ζ����յ㣬��Na2S2O3��Һ30.00 mL��
�ٲ���������������⻯�ط�Ӧ�����ӷ���ʽΪ ��
�ڷ�Ӧ�����Һ�д������ƺ������Ƶ����ʵ���֮��Ϊ ��
�ۼ�������������Һ�����ʵ���Ũ�ȡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
Cl2�Ƿ�֯��ҵ�г��õ�Ư����Na2S2O3����ΪƯ�ײ�ƥ��ġ����ȼ����� S2O32-��Cl2��Ӧ�IJ���֮һΪSO42һ������˵���У�������� ( )
| A���÷�Ӧ�е���������C12 |
| B��SO2����ˮ��Ư��ԭ����ͬ�����Կ���S02����֯��ҵ��Ư�� |
| C��������Ӧ�У�ÿ����1 mol SO42һ������ȥ2 mol C12 |
| D�����ݸ÷�Ӧ���жϻ�ԭ�ԣ�S2O32->C1�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����й�������ԭ��Ӧ����������ȷ���� �� ��
| A��������ԭ��Ӧ�ı����ǵ��ӵ�ת�� |
| B����������ԭ��Ӧ�У�ʧȥ���ӵ����ʣ�һ����Ԫ�ػ��ϼ����� |
| C���϶���һ��Ԫ�ر���������һ��Ԫ�ر���ԭ |
| D���ڷ�Ӧ�в�һ������Ԫ�صĻ��ϼ۶������仯 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com