��������1�����ݿ�ʼʱ����������ʵ������������Ũ�ȣ�
��2�����õ�Ԫ���غ������������ʵ������ٸ���V=nV
m������������������
��3����Ӧ��������Һ������Ϊ����ͭ�������������������n��Cu����
��4��������Ӧ��3Cu+2NO
3-+8H
+=3Cu
2++2NO��+4H
2O���ݴ˼���������������ʵ���������������Ҫ����������
��5���ɷ���ʽ2NO
2+2NaOH=NaNO
2+NaNO
3+H
2O��NO+NO
2+2NaOH=2NaNO
2+H
2O��֪��NO�������ܱ����գ�NO��NO
2������屻NaOH��Һ����ȫ���գ�����n��NO
2����n��NO����1����n��NO
2����n��NO��=1ʱxֵ��С������x����Сֵ����Ϊ����NO������x���ֵ��2���ݴ�ȷ��xȡֵ��Χ��
�ݹ��������̣�Cuʧȥ�ĵ��ӵ�������������������ʱ��õĵ��ӣ����ݵ���ת���غ����n��NaNO
2��������NԪ���غ����n��NaNO
3����
��6���ڳ����£���NO����ѹ����1.01��10
7Pa���ټ��ȵ�50�棬���������ѹǿѸ���½���ѹǿ������С��ԭѹǿ��
��Ȼ��ѹǿ�Ͳ��ٸı䣮��֪����һ�ֲ���ΪN
2O������ԭ�Ӹ����غ��֪�÷�ӦΪ��3NO=N
2OʮNO
2��������2NO
2
N
2O
4�������ɵ�NO
2��˫�۳�N
2O
4������������������٣�ѹǿ������С��ԭѹǿ��
��
��ֻ������Ӧ��3NO=N
2OʮNO
2�������ƽ��Ħ��������С��������������ȫת��Ϊ����������ʱ�������ƽ��Ħ��������ݴ˼�����
���
�⣺��1����ͼ��֪����ʼʱn��NO
3-��=1.0mol����n��HNO
3��=n��NO
3-��=1.0mol����c��HNO
3��=
=10mol/L���ʴ�Ϊ��10��
��2����ͼ��֪����Ӧ����ʱ����Һ��NO
3-Ϊ0.6mol�����ݵ�Ԫ���غ㣬������������ʵ���Ϊ1.0mol-0.6mol=0.4mol������¶������������Ϊ0.4mol��22.4L/mol=8.96L���ʴ�Ϊ��8.96��
��3����Һ������Ϊ����ͭ����ͼ��֪����Ӧ����ʱ����Һ��NO
3-Ϊ0.6mol��������n��Cu��=n������ͭ��=
=0.3mol���ʴ�Ϊ��0.3��
��4��28.8gͭ�����ʵ���Ϊ
=0.45mol����ʣ��CuΪ0.45mol-0.3mol=0.15mol��
3 Cu+2 NO
3-+8 H
+=3Cu
2++2NO��+4H
2O
3 2 8
0.15mol n��H
+��
��n��H
+��=
=0.4mol����n��H
2SO
4��=0.2mol������Ҫ�������Ϊ
=0.1L=100mL��
�ʴ�Ϊ��100��
��5���ɷ���ʽ��֪��NO�������ܱ����գ�NO��NO
2������屻NaOH��Һ����ȫ���գ�����n��NO
2����n��NO����1����n��NO
2����n��NO��=1ʱxֵ��С��x��СֵΪ
=1.5����Ϊ����NO������x���ֵ��2����x��ȡֵ��ΧΪ1.5��x��2��
�ݹ��������̣�Cuʧȥ�ĵ��ӵ�������������������ʱ��õĵ��ӣ����ݵ���ת���غ㣬n��NaNO
2��=n��Cu��=
=
mol������NԪ���غ㣬��֪n��NaNO
3��=n�����壩=
-
mol=��
-
��mol��
�ʴ�Ϊ��1.5��x��2��
����
-
����
��6���ڳ����£���NO����ѹ����1.01��10
7Pa���ټ��ȵ�50�棬���������ѹǿѸ���½���ѹǿ������С��ԭѹǿ��
��Ȼ��ѹǿ�Ͳ��ٸı䣮��֪����һ�ֲ���ΪN
2O������ԭ�Ӹ����غ��֪�÷�ӦΪ��3NO=N
2OʮNO
2��������2NO
2
N
2O
4�������ɵ�NO
2��˫�۳�N
2O
4������������������٣�ѹǿ������С��ԭѹǿ��
��
��ֻ������Ӧ��3NO=N
2OʮNO
2�������ƽ��Ħ��������С����ʱM=
=45g/mol��
������������ȫת��Ϊ����������ʱ�������ƽ��Ħ���������1molNO
2�õ�0.5molN
2O
4��
���ʱM=
| 1mol��44g/mol+0.5mol��92g/mol |
| 1mol+0.5mol |
=60g/mol��
���Ϸ�������֪45g/mol��M��60g/mol��
�ʴ�Ϊ��3NO=N
2OʮNO
2��2NO
2
N
2O
4��45g/mol��M��60g/mol��

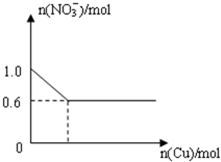 ��28.8gͭͶ��100mLŨ�����У����ͭ��ʣ�࣬��NO3-�������ʵ����仯��ͼ��ʾ����ش��������⣺
��28.8gͭͶ��100mLŨ�����У����ͭ��ʣ�࣬��NO3-�������ʵ����仯��ͼ��ʾ����ش��������⣺ N2O4�������ɵ�NO2��˫�۳�N2O4������������������٣�ѹǿ������С��ԭѹǿ��
N2O4�������ɵ�NO2��˫�۳�N2O4������������������٣�ѹǿ������С��ԭѹǿ�� N2O4�������ɵ�NO2��˫�۳�N2O4������������������٣�ѹǿ������С��ԭѹǿ��
N2O4�������ɵ�NO2��˫�۳�N2O4������������������٣�ѹǿ������С��ԭѹǿ�� N2O4��45g/mol��M��60g/mol��
N2O4��45g/mol��M��60g/mol��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
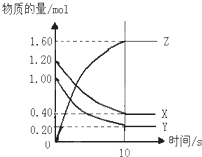 ����ͼ��ʾ��ij�¶��£����ʵ����ֱ���1.2mol������X�����ʵ���Ϊ1.0mol������Y����2L�ܱ������з�Ӧ��������Z����Ӧ5min����n��X��=0.4mol��n��Y��=0.2mol�����ɵ�n��Z��=1.6mol����÷�Ӧ�Ļ�ѧ����ʽ�ɱ�ʾΪ
����ͼ��ʾ��ij�¶��£����ʵ����ֱ���1.2mol������X�����ʵ���Ϊ1.0mol������Y����2L�ܱ������з�Ӧ��������Z����Ӧ5min����n��X��=0.4mol��n��Y��=0.2mol�����ɵ�n��Z��=1.6mol����÷�Ӧ�Ļ�ѧ����ʽ�ɱ�ʾΪ