
����Ŀ�������������壨FeC2O4��2H2O����һ�ֻ�ɫ������ˮ�Ĺ��壬�����ֽ⣬��������ء�Ϳ���Լ��й���ϵ�ԭ���ϡ�Ϊ̽�������������������ȷֽ�IJ�����װ��ͼ���£�
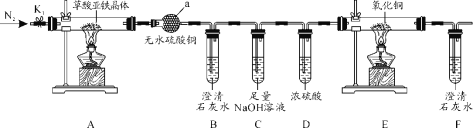
��1������a��������______��
��2������ɫ��ѧ���ǣ�����װ�ô��ڵ�����ȱ����_________��
��3��ʵ��ǰ��ͨ��һ��ʱ��N2����Ŀ��Ϊ__________��
��4��ʵ��֤������������к���CO�����ݵ�ʵ������Ϊ_______��
��5���������������ڿ����ױ�����������������������Ƿ��������ʵ�ʵ�������____��
��6����ȡ5.40g�����������������ط���������ȷֽ⣬�õ�ʣ������������¶ȱ仯����������ͼ��ʾ��
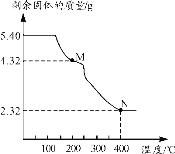
����ͼ��M���Ӧ���ʵĻ�ѧʽΪ_________��
����֪400��ʱ��ʣ�����������һ���������ͨ������д��M��N������Ӧ�Ļ�ѧ����ʽ��_______��
���𰸡����θ���ܣ�����ܣ� ȱ�ٴ���COβ��װ�� �ž�װ���ڿ�������ֹ������H2O��CO2�ĸ��� E�к�ɫ��ĩ���ɫ��F���ְ�ɫ���� ȡ�������������������Թ��У�����ϡ�����ܽ�μ�KSCN��Һ������Һ���ɫ������������������������ʣ��������ɫ���������������δ�������� FeC2O4 3FeC2O4 ![]() Fe3O4��2CO2����4CO��
Fe3O4��2CO2����4CO��
��������
װ��AΪ������������ֽ⣬������ˮ����ͭ����ˮ������Bװ�ü��������̼��Cװ�����ն�����̼��Dװ�ø������壬Eװ�ü���CO��Fװ�ü��������̼���ݴ˽��
(1)����a�����������θ����(�����)���ʴ�Ϊ�����θ����(�����)��
(2)��Ӧ�����CO��ȱ�ٴ���COβ��װ�ã��ʴ�Ϊ��ȱ�ٴ���COβ��װ�ã�
(3)��Ӧ������CO��H2O��ͨ�뵪�����ž�װ���ڿ�������ֹ������H2O��CO2�ĸ��ţ��ʴ�Ϊ���ž�װ���ڿ�������ֹ������H2O��CO2�ĸ��ţ�
(4)CO��CuO��Ӧ������Cu�Ͷ�����̼������ΪE�к�ɫ��ĩ���ɫ��F���ְ�ɫ�������ʴ�Ϊ��E�к�ɫ��ĩ���ɫ��F���ְ�ɫ������
(5)���������ӵ��Լ�ΪKSCN�������У�ȡ�������������������Թ��У�����ϡ�����ܽ�μ�KSCN��Һ������Һ���ɫ������������������������ʣ��������ɫ���������������δ�������ʣ��ʴ�Ϊ��ȡ�������������������Թ��У�����ϡ�����ܽ�μ�KSCN��Һ������Һ���ɫ������������������������ʣ��������ɫ���������������δ�������ʣ�
(6)�ٲ���������������ʵ���Ϊ��![]() ��0.03mol��ͨ��ʣ����������Ϊ4.32g����M��Ħ������Ϊ
��0.03mol��ͨ��ʣ����������Ϊ4.32g����M��Ħ������Ϊ![]() ��144g/mol�����̢����ķ�Ӧ�ǣ�����������������ʧȥ�ᾧˮ��ʣ�µ�MΪFeC2O4���ʴ�Ϊ��FeC2O4��
��144g/mol�����̢����ķ�Ӧ�ǣ�����������������ʧȥ�ᾧˮ��ʣ�µ�MΪFeC2O4���ʴ�Ϊ��FeC2O4��
�ڲ������������е���Ԫ������Ϊ��![]() ��1.68g���������������е���Ԫ����ȫת�����������У�����������Ԫ�ص�����Ϊ��
��1.68g���������������е���Ԫ����ȫת�����������У�����������Ԫ�ص�����Ϊ��![]() ��������������Ļ�ѧʽΪFexOy�����У�
��������������Ļ�ѧʽΪFexOy�����У�![]() �����x��y��3��4������������Ļ�ѧʽΪFe3O4�����M��N������Ӧ�Ļ�ѧ����ʽΪ3FeC2O4
�����x��y��3��4������������Ļ�ѧʽΪFe3O4�����M��N������Ӧ�Ļ�ѧ����ʽΪ3FeC2O4 ![]() Fe3O4��2CO2����4CO����
Fe3O4��2CO2����4CO����


 ������ϵ�д�
������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ɫ��Һ�к��е�������ΪH+��Na+��Mg2+��Al3+��Ba2+�е�һ�ֻ��֣������Һ�л����ص���NaOH��Һֱ���������������������������NaOH��Һ������Ĺ�ϵ��ͼ��ʾ���ɴ�ȷ��ԭ��Һ��һ�����е���������
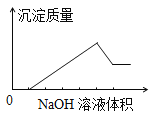
A. Mg2+��Al3+��Na+ B. H+��Mg2+��Al3+ C. H+��Ba2+��Al3+ D. Ba2+��Mg2+��Al3+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ϳɰ��������������ú��ˮ��ԭ�Ͼ��ಽ��Ӧ�Ƶã����е�һ����ӦΪ��CO(g)��H2O(g) ![]() CO2(g)��H2(g) ��H<0����Ӧ�ﵽƽ���Ϊ���CO��ת���ʣ����д�ʩ����ȷ����( )
CO2(g)��H2(g) ��H<0����Ӧ�ﵽƽ���Ϊ���CO��ת���ʣ����д�ʩ����ȷ����( )
A.����CO��Ũ��B.�����¶�C.�����¶�D.����ѹǿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʵ��������������ԭ�������͵���
A.���ű���ʳ��ˮ�ķ����ռ�����
B.�ϳɰ���ҵѡ�����
C.����ơ��ƿ��ƿ�����Ϸ��������ĭ
D.��Fe3++3SCN-![]() Fe(SCN)3��Ӧ��ƽ��ʱ������KSCN��Ũ�ȣ���ϵ��ɫ����
Fe(SCN)3��Ӧ��ƽ��ʱ������KSCN��Ũ�ȣ���ϵ��ɫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ�������������ء�������������Ѫ�쵰�ס����쵰����O2��ϻ��Ƶ�����о����ٶ��价���¶Ⱦ�Ϊ36.8�档
(1)Ѫ�쵰��Hb���O2�γɶ���Ѫ�����ڷ�Ӧ�٣�HbH+(aq)+O2(g)![]() HbO2(aq)+H+(aq)���÷�Ӧ���Է����У�������H______0(���������)��ѪҺ�л�ͬʱ���ڷ�Ӧ�ڣ�CO2+H2O
HbO2(aq)+H+(aq)���÷�Ӧ���Է����У�������H______0(���������)��ѪҺ�л�ͬʱ���ڷ�Ӧ�ڣ�CO2+H2O![]() H++HCO3-����Ϸ�Ӧ�٢ڣ��β�����ѹ_____(��ϸߡ��ϵ͡�)������CO2�ų����⣬�ӻ�ѧƽ��ǶȽ���ԭ�� ____________��
H++HCO3-����Ϸ�Ӧ�٢ڣ��β�����ѹ_____(��ϸߡ��ϵ͡�)������CO2�ų����⣬�ӻ�ѧƽ��ǶȽ���ԭ�� ____________��
(2)�����д������쵰�� MbҲ�ɽ��O2�γ�MbO2������Ӧ�ۣ�Mb(aq)+O2(g)![]() MbO2(aq)����ƽ�ⳣ��K=
MbO2(aq)����ƽ�ⳣ��K=![]() �������������䣬��������ѹp(O2)����Kֵ___(��������С�����䡱)����֪������ѹp(O2)=2.00 kPa ��ƽ����ϵ�У�
�������������䣬��������ѹp(O2)����Kֵ___(��������С�����䡱)����֪������ѹp(O2)=2.00 kPa ��ƽ����ϵ�У�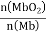 =4.0������Ŀ�����p(O2)=21 kPa�������ʱ Mb������������϶�(ƽ��ת����)ԼΪ_______________(������λ��Ч����)��
=4.0������Ŀ�����p(O2)=21 kPa�������ʱ Mb������������϶�(ƽ��ת����)ԼΪ_______________(������λ��Ч����)��
(3)Hb���Ӿ����ĸ��ǻ�����ÿ���ǻ������ֹ���(T�ͺ�R��)��ͼ�У�T0��R0��ʾδ���O2��T�ͺ�R�ͣ��Ҵ��ڿ���ı乹ЧӦ��T0![]() R0������ƽ�ⳣ��ΪK0�����ķ���O2��Hb���ĸ��ǻ���Ϻ�T4
R0������ƽ�ⳣ��ΪK0�����ķ���O2��Hb���ĸ��ǻ���Ϻ�T4![]() R4Ҳ�DZ乹ЧӦ������ƽ�ⳣ��ΪK4��
R4Ҳ�DZ乹ЧӦ������ƽ�ⳣ��ΪK4��
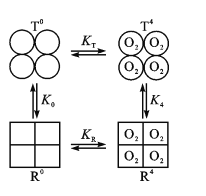
����֪ij���ײ��˷�����T0+4O2![]() T4��Ӧ��n(O2)�������£�
T4��Ӧ��n(O2)�������£�
t/min | 0 | 2.0 | 4.0 | 6.0 | 8.0 |
n(O2)/10-6 mol | 1.68 | 1.64 | 1.58 | 1.50 | 1.40 |
����2.0 min~8.0 min����T�����ʵ����仯��ʾ�ķ�Ӧ����v(T4)Ϊ_________mol��min-1��
���ּٶ�R��Hb��O2�Ľ�ϳ���ΪKR��T��Hb��O2�Ľ�ϳ���ΪKT����֪KR��KT����ͼ��K0____K4(���������)��
(4)������������ز����ٵ����ʡ�����ͼ��ʾ����PtΪ������Pb(CO2)�����壬ʹCO2�Ϊ��������⾭CO2���ʹ�����KHCO3��Һ��ʹ����������ͬʱ�õ��״�����������ӦʽΪ____���ӵ�����Һ�з���״��IJ���������_______________��
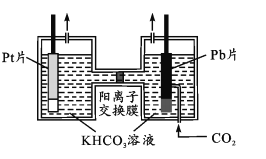
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й�ʵ��װ����ȷ���ܴﵽʵ��Ŀ�ĵ��ǣ� ��
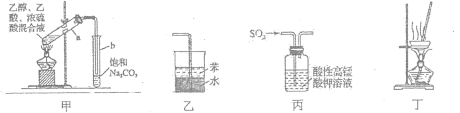
A.��ͼ����ʾװ����ȡ������������
B.��ͼ����ʾװ�����հ���������
C.��ͼ����ʾװ����֤SO2��Ư����
D.��ͼ����ʾװ������NH4Cl������Һ�Ʊ�NH4Cl����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ��ȤС���ú�A��B���ֽ������ʵķ�ĩ״������������ʵ�飬��ת����ϵ����ͼ��ʾ�����ַ�Ӧ���������δ�г���������EΪ��ɫ��״������IΪ���ɫ����������ת����ϵ�����õ��Լ����������ģ�

��1��д���������ʵĻ�ѧʽ��F____________��G________________��
��2��������������ֽ������뿪����ķ�����___________��
��3��D��E��ת���У����������X������_____________________��
A.����NaCl��Һ B.NaOH��Һ C.��ˮ D.Ba(OH)2��Һ
��4��д������ת���Ļ�ѧ����ʽ��
A��C��______________________________________________��
H��I��_______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��N2O5��һ��������������һ���¶��·���2N2O5(g)![]() 4NO2(g)��O2(g)����H>0��T1�¶��µIJ���ʵ������Ϊ
4NO2(g)��O2(g)����H>0��T1�¶��µIJ���ʵ������Ϊ
t/s | 0 | 500 | 1 000 | 1 500 |
c(N2O5)mol/L | 5.00 | 3.52 | 2.50 | 2.50 |
����˵������ȷ������ ��
A. 500 s��N2O5�ֽ�����Ϊ2.96��10��3 mol/(L��s)
B. T1�¶��µ�ƽ�ⳣ��ΪK1��125��1 000 sʱת����Ϊ50%
C. ������������ʱ��T2�¶��·�Ӧ��1 000 sʱ���N2O5(g)Ũ��Ϊ2.98 mol/L����T1<T2
D. T1�¶��µ�ƽ�ⳣ��ΪK1��T2�¶��µ�ƽ�ⳣ��ΪK2����T1>T2����K1>K2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��NaHSO4ͨ��Ϊ���壬������ˮ���Իش��������⣺
��1��NaHSO4�����ʷ���������________��
A ���� B ������ C ��ʽ�� D ������
д��NaHSO4������״̬�µĵ��뷽��ʽ_____________��NaHSO4ˮ��Һ�ܷ���Mg��Ӧ?________��������������������������ܣ�д�����ӷ���ʽ��_________��������ܴ��ʲ�����
��2��ijͬѧ̽����Һ�ĵ�����������Ũ�ȵĹ�ϵ������ʵ�飬�ζ��������ڵμ���Һ��������

�����ձ�����ʢҺ����NaHSO4��Һ���ζ�������Ba��OH��2��Һ������Ba��OH��2��Һ�ĵμӣ������䰵�����μ�����Һ������ʱ�����ӷ���ʽ��_______�������μӣ�������Ӧ�����ӷ���ʽΪ��__________��
������������Ͽ�ʹ�μӹ����е����ȱ�����䰵_______
A ������еμ���ͬŨ�ȵİ�ˮ
B ʯ�����еμ�ϡ����
C �������еμ���ͬŨ�ȵ�����������Һ
D ���������Һ�еμ���ͬŨ�ȵ�����������
������NaHSO4����NH4HSO4��Һ������������Ba��OH��2��Һ��������Ӧ�����ӷ���ʽΪ__________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com