
����Ŀ���й����ʵ����ļ�������ѧ��ѧ����Ҫ���ݣ����������գ�
��1����״���£���6.72 L NH3 ��1.204��1023�� H2S ��5.6 g CH4 ��0.5 mol HCl �����й�ϵ���ɴ�С����
A���������___________________ B����������________________
C���ܶȴ�С��___________________D��ԭ����Ŀ��_________________
��2����״���£�33.6 L��HCl�����е����ʵ���Ϊ_____________�������ܽ���ˮ���3 L����Һ��������������ʵ���Ũ��Ϊ__________��
��3����25 �桢101 kPa�������£�ͬ������CH4��A��������֮����15��8����A��Ħ������Ϊ________
��4����״���£�2.4 gij��������Ϊ672 mL������������Է�������Ϊ_________
��5��0.5 L 1 mol/L CaCl2��Һ��Ca2+�����ʵ���Ϊ_________��Cl�����ʵ���Ũ��Ϊ________
��6��0.3 mol NH3����������ԭ������___________��H2O����������ԭ�������
��ͬ������SO2��SO3�����ʵ���֮��Ϊ____________����ԭ�ӵĸ���֮��Ϊ___________
��0���101kPa�������£���2.00g������1.40g������1.60g������ϣ��û������������________L
��1gN2��a��ԭ�ӣ����ӵ������ɱ�ʾΪ_____________
������ء���������������ɵĻ����Һ������c(H��)��0.1 mol/L��c(Fe3��)��0.3 mol/L��c(SO![]() )�� 0.6 mol/L����c(K��)Ϊ________
)�� 0.6 mol/L����c(K��)Ϊ________
���𰸡���>��>��>�ڢ�>��>��>�٢�>��>��>�ۢ�>��>��>��1.5 mol0.5 mol/L30g/mol800.5 mol2 mol/L0.4NA5��45��613.44L28a mol-10.2mol/L
��������
��1����״���£���6.72LNH3�����ʵ���Ϊ6.72L��22.4L/mol==0.3mol����1.204��1023��H2S�����ʵ���Ϊ1.204��1023��6.02��1023mol1==0.2mol����5.6gCH4�����ʵ���Ϊ5.6g��16g/mol=0.35mol����0.5molHCl��A.�����������֪���ʵ����������������ڣ���ͬ�����£����֮�ȵ������ʵ���֮�ȣ����������С����������������B.��NH3����Ϊ17g/mol��0.3mol=5.1g����H2S����Ϊ34g/mol��0.2mol=6.8g����CH4��Ϊ5.6g����HCl����Ϊ36.5g/mol��0.5mol=18.25g����������С����������������C.ͬ��ͬѹ�£��ܶ�֮�ȵ�����Է�������֮�ȣ���NH3��Է�������Ϊ17����H2S��Է�������Ϊ34����CH4��Է�������Ϊ16��HCl��Է�������Ϊ36.5�����ܶȴ�С����������������D.�ٱ�״��6.72LNH3��ԭ�ӵ����ʵ���Ϊ0.3mol��4=1.2mol����1.204��1023��H2S���е�ԭ�ӵ����ʵ���Ϊ0.2mol��3=0.6mol����5.6gCH4���е�ԭ�ӵ����ʵ���Ϊ0.35mol��5=1.75mol����0.5molHCl���е�ԭ�ӵ����ʵ���Ϊ0.5mol��2=1mol��ԭ����Ŀ֮�ȵ������ʵ���֮�ȣ�����ԭ����Ŀ����������������
��2��33.6 L��HCl�����ʵ���Ϊ��33.6 L��22.4L/mol=1.5mol�����γ���Һ��Ũ��c=1.5mol��3L=0.5mol/L���ʴ�Ϊ��1.5 mol�� 0.5 mol/L��
��3������ͬ�����£�ͬ������������������֮�ȵ���Ħ�������ķ��ȣ�����V(CH4)��V(A)��M(A)��M(CH4)����15��8��M(A)��16 g/mol����M(A)��30 g/mol���ʴ�Ϊ��30 g/mol��
��4��672ml��������ʵ���Ϊ:672��10-3L��22.4L/mol=0.03mol������Ħ������Ϊ��2.4g��0.03mol=80g/mol���ʴ�Ϊ��80��
��5��0.5 L 1 mol/L CaCl2�����ʵ���Ϊ��0.5 L�� 1 mol/L=0.5mol��Ca2+�����ʵ���Ϊ0.5mol��Cl�����ʵ���Ũ��Ϊ1 mol/L��2=2mol/L���ʴ�Ϊ��0.5 mol�� 2 mol/L��
��6��0.3 mol NH3����������ԭ�ӵ����ʵ���Ϊ:0.3mol��4=1.2mol����֮ԭ������ȵ�H2O���ʵ���Ϊ��1.2mol��3=0.4mol���������Ӹ���Ϊ0.4NA����ͬ������SO2��SO3�����ʵ���֮��Ϊ80:64=5��4����ԭ�Ӹ���֮��Ϊ��5��2:4��3=5��6��2.00g���������ʵ���=2.00g��4g/mol=0.5mol��1.40g���������ʵ���Ϊ��1.40g��28g/mol=0.05mol��1.60g���������ʵ���Ϊ��1.60g��32g/mol=0.05mol��������������ʵ���Ϊ0.5mol+0.05mol+0.05mol=0.6mol�������Ϊ��0.6mol��22.4L/mol=13.44L����1gN2��a��ԭ�ӣ�����![]() ��NA=28amol-��������Һ�еĵ���غ�ɵñ���ʽ��c(K��)+c(H��)+3c(Fe3��)=2c(SO
��NA=28amol-��������Һ�еĵ���غ�ɵñ���ʽ��c(K��)+c(H��)+3c(Fe3��)=2c(SO![]() )��c(K��)= 0.2mol/L���ʴ�Ϊ��0.4NA��5��4�� 5��6��13.44L�� 28a mol-1 ��0.2mol/L��
)��c(K��)= 0.2mol/L���ʴ�Ϊ��0.4NA��5��4�� 5��6��13.44L�� 28a mol-1 ��0.2mol/L��


 ��ɢ˼ά�¿���ϵ�д�
��ɢ˼ά�¿���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ҫ��������и�С��
��1��д��NaHSO4��ˮ��Һ�еĵ��뷽��ʽ______________________________________��
��2���Ȼ�����ˮ��Һ����ʱ��_____(��ᡱ�����С������)�ԣ����Ȼ�����Һ���ɣ����գ����õ��Ĺ��������__________��
��3��ʵ��������FeSO4��Һ���ܽ�ʱ��Ҫ����������ϡ���ᣬ��ԭ����___________________(�����ӷ���ʽ���ʵ�����˵��)��������Ϻ�Ҫ����������м����Ŀ����____________________________��
��4��t��ʱ��ijNaOHϡ��Һ��c(H+)=10��a mol��L��1��c(OH��)=10��b mol��L��1����֪a+b=12����
�ٸ��¶���ˮ�����ӻ�����Kw=________________��
���ڸ��¶��£���100mL 0.1 mol��L��1��ϡH2SO4��100mL 0.4 mol��L��1��NaOH��Һ��Ϻ���Һ��pH= _____________��
���𰸡� NaHSO4=Na++H++SO42- �� Al2O3 Fe2++2H2OFe(OH)2+2H+ ����Fe2+ˮ�� ��ֹFe2+���� 1.0��10-12 11
����������1��. NaHSO4��ǿ�����ʽ�Σ���ˮ��Һ����ȫ���룬���뷽��ʽΪ��NaHSO4=Na++H++SO42-���ʴ�Ϊ��NaHSO4=Na++H++SO42-��
��2��.AlCl3��ǿ�������Σ�ˮ��ʹ��Һ�����ԣ�Al3����3H2O![]() Al(OH)3��3H�������������Ȼ�����Һ���ٽ������ӵ�ˮ�⣬ʹƽ�������ƶ�����HCl�ӷ��������ɺ�õ�Al(OH)3����������ʱAl(OH)3�����ֽ���2Al(OH)3
Al(OH)3��3H�������������Ȼ�����Һ���ٽ������ӵ�ˮ�⣬ʹƽ�������ƶ�����HCl�ӷ��������ɺ�õ�Al(OH)3����������ʱAl(OH)3�����ֽ���2Al(OH)3 ![]() Al2O3��3H2O���������պ�õ����������壬�ʴ�Ϊ������Al2O3��
Al2O3��3H2O���������պ�õ����������壬�ʴ�Ϊ������Al2O3��
��3��.ʵ��������FeSO4��Һ�����������ӷ���ˮ����Fe2++2H2O![]() Fe(OH)2+2H+�����ܽ�ʱ�ȼ���������ϡ���ᣬ����������Ũ�ȣ�����Fe2+ˮ�⣻��Fe2+���ױ������е���������ΪFe3����������Ϻ����������м�����Է�����Fe��2Fe3��=3Fe2�����Ӷ��ﵽ��ֹFe2+�����������ã��ʴ�Ϊ��Fe2++2H2O
Fe(OH)2+2H+�����ܽ�ʱ�ȼ���������ϡ���ᣬ����������Ũ�ȣ�����Fe2+ˮ�⣻��Fe2+���ױ������е���������ΪFe3����������Ϻ����������м�����Է�����Fe��2Fe3��=3Fe2�����Ӷ��ﵽ��ֹFe2+�����������ã��ʴ�Ϊ��Fe2++2H2O![]() Fe(OH)2+2H+������Fe2+ˮ������ֹFe2+������
Fe(OH)2+2H+������Fe2+ˮ������ֹFe2+������
��4��.�� . t��ʱ��ijNaOHϡ��Һ��c(H+)=10��a mol��L��1��c(OH��)=10��b mol��L��1����Kw= c(H+)��c(OH��)= 10��a mol��L��1��10��b mol��L��1=1.0��10��(a+b)����֪a+b=12����Kw=1.0��10��12���ʴ�Ϊ��1.0��10��12��
���ڸ��¶��£�100mL 0.1 mol��L��1��ϡH2SO4��Һ��n��H+��=0.1L��0.1 mol��L��1��2=0.02mol��100mL 0.4 mol��L��1��NaOH��Һ��n��OH����=0.1L��0.4 mol��L��1=0.04mol������Һ��Ϻ����������ӹ�����������Һ��c��OH����=![]() = 0.1mol/L����c��H+��=
= 0.1mol/L����c��H+��=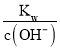 =10��11mol/L����pH= ��lgc��H+��=11���ʴ�Ϊ��11��
=10��11mol/L����pH= ��lgc��H+��=11���ʴ�Ϊ��11��
�����͡�������
��������
24
����Ŀ����֪25 ��ʱ,����������ʵĵ���ƽ�ⳣ���������±���
���ữѧʽ | CH3COOH | HCN | H2CO3 |
����ƽ�ⳣ�� | 1.7��10��5 | 6.2��10��10 | K1��4.3��10��7 K2��5.6��10��11 |
��1�������ӷ���ʽ��ʾNa2CO3��Һ�ʼ��Ե�ԭ��____________________��
��2�������ʵ���Ũ�ȵ�A��CH3COONa B��NaCN C��Na2CO3 D��NaHCO3��Һ��pH�ɴ�С��˳��Ϊ____________________________________(����ĸ)��
��3����֪��25��ʱ, ��HCN��Һ��NaOH��Һ�������Ũ�Ȼ�Ϻ�,����Һ������Ũ���ɴ�С��˳����____________________________________��
��4�������£�0.1mol��L��1��CH3COOH��Һ��ˮϡ�ͣ����б���ʽ�����ݱ�����______��
A��c(H��) B��c(H��)/c(CH3COOH) C��c(H��)��c(OH��)
��5�������Ϊ10 mL ,pH��Ϊ2�Ĵ�����Һ������ֱ�������Zn��Ӧ����Ӧ�տ�ʼʱ����H2�����ʣ�v(HCl)______v(CH3COOH)���������������������ͬ������Ӧ��ȫ������������������m(H2)����_______m(H2)������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±�ΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã��ش��������⣺
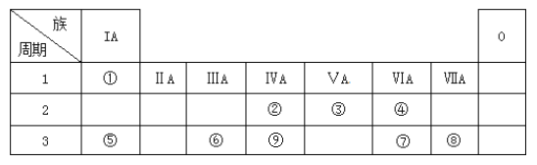
��1������������Ԫ�طǽ�������ǿ��Ԫ�ص�ԭ�ӽṹʾ��ͼΪ__________��
��2���ڢۢ�����������Ӧˮ��������ǿ��˳��Ϊ���ѧʽ��_____________��
��3���õ���ʽ��ʾ�ܵ��⻯����γɹ���_________________________��
��4�����п����жϢݺ͢�����ǿ������__________��
a. �ݵ��ʵ��۵�Ȣ��ʵ�
b. �ݵĻ��ϼ۱Ȣ�
c. �ݵ�����ˮ��Ӧ�ȵ��ʢ���
d. ������������ˮ����ļ��ԱȢ�ǿ
��5���ɱ��Т١��ۡ��ܡ��ޡ���Ԫ���γɵij�������Z��M��N�ɷ������·�Ӧ��
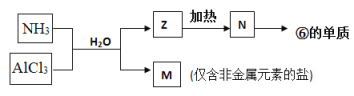
a. M�������Ļ�ѧ������Ϊ���������ۼ�����������Ի�Ǽ��ԣ�_________��
b. N���ĵ��ʵĻ�ѧ����ʽ_________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ���¶��£����ݻ�Ϊ2 L�ĺ����ܱ������г���6molCO2 ��8molH2��������ӦCO2(g)+3H2(g) ![]() CH3OH(g)+H2O(g) ��H= -49.0kJmol-1�����n(H2)��ʱ��ı仯�����ߢ���ʾ������˵����ȷ����
CH3OH(g)+H2O(g) ��H= -49.0kJmol-1�����n(H2)��ʱ��ı仯�����ߢ���ʾ������˵����ȷ����
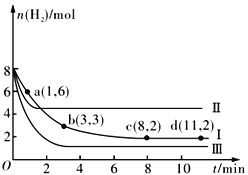
A. �÷�Ӧ��0~8 min��CO2 ��ƽ����Ӧ������0.375mol��L-1��min-1
B. �����¶Ȳ��䣬����ʼʱ�����������г���4molCO2��2molH2��2molCH3OH(g)��1mol H2O(g)�����ʱ��Ӧ������Ӧ�������
C. �����¶Ȳ��䣬����ʼʱ�����������г���3molCO2 ��4molH2����ƽ��ʱH2 �������������20%
D. �ı������õ����ߢ������ߢ�ı�������ֱ��������¶ȡ����뺤��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Cu2O���ھ��������Ĵ����ܶ��ܵ���ע���±�Ϊ��ȡCu2O�����ַ�����
����Cu2O���ھ��������Ĵ����ܶ��ܵ���ע���±�Ϊ��ȡCu2O�����ַ�����
����a | ��̿���ڸ��������»�ԭCuO |
����b | ��ⷨ����ӦΪ2Cu + H2O |
����c | ���£�N2H4����ԭ����Cu(OH)2 |
��1����֪��2Cu(s)��![]() O2(g)=Cu2O(s)��H =-169kJ��mol-1
O2(g)=Cu2O(s)��H =-169kJ��mol-1
C(s)��![]() O2(g)=CO(g)��H =-110.5kJ��mol-1
O2(g)=CO(g)��H =-110.5kJ��mol-1
Cu(s)��![]() O2(g)=CuO(s)��H =-157kJ��mol-1
O2(g)=CuO(s)��H =-157kJ��mol-1
��a������Ӧ���Ȼ�ѧ����ʽ��_____________________________________��
��2������b�������ӽ���Ĥ���Ƶ��Һ��OH-��Ũ�ȶ��Ʊ�����Cu2O��װ����ͼ��ʾ�������ӽ���ĤΪ______���ӽ���Ĥ(�����������),�õ�ص�������ӦʽΪ______________________________________��

��3������cΪ������������Һ̬�£�N2H4����ԭ����Cu(OH)2���Ʊ�����Cu2O��ͬʱ�ų�N2�����Ʒ��Ļ�ѧ����ʽΪ________________________________________��
��4�����ݻ�Ϊ1L�ĺ����ܱ������У������Ϸ����Ƶõ���������Cu2O�ֱ���д��ֽ�ˮ��ʵ�飺2H2O(g) ![]() 2H2(g)+O2(g)����H>0��ˮ������Ũ��c��ʱ��t�ı仯���±���ʾ��
2H2(g)+O2(g)����H>0��ˮ������Ũ��c��ʱ��t�ı仯���±���ʾ��

�ٶԱ�ʵ����¶ȣ�T2_________T1�����������������=����
�ڴ�����Ч�ʣ�ʵ���________ʵ��ڣ������������
����ʵ��۴ﵽƽ��״̬�����������ͨ��ˮ������������0.1mol����Ӧ�ٴδﵽƽ��ʱ��������������Ũ��Ϊ ____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ����������������ȷֽ�Ļ�ѧ����ʽΪ��5NH4NO3![]() 2HNO3��4N2��9H2O���ڷ�Ӧ�б������뱻��ԭ�ĵ�ԭ����֮��Ϊ( )
2HNO3��4N2��9H2O���ڷ�Ӧ�б������뱻��ԭ�ĵ�ԭ����֮��Ϊ( )
A. 5��3 B. 5��4 C. 1��1 D. 3��5
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A~J����ѧ��ѧ���������ʣ�����֮���ת����ϵ����ͼ��ʾ �����ַ�Ӧ��������������ʡ�ԣ�����֪A��һ�ָ��۵����ʣ�D��һ�ֺ���ɫ���塣
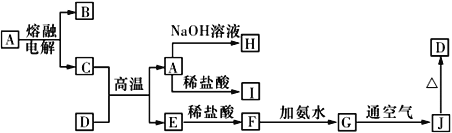
��ش��������⣺
��1�����A���ʵ������缫��ӦʽΪ________��C��D�ڸ����·�Ӧ�������÷�Ӧ��Ҫ����������Լ���________________________�������ƣ���
��2��д��G��J��Ӧ��ʵ�������뻯ѧ����ʽ��____________________________��________________________________��
��3��C��NaOH��Һ��Ӧ�����ӷ���ʽΪ________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)������������HNO2��Ӧ�õ����ǻ��ᡣ�磺

��д�����б仯��A��B��C��D�����л���Ľṹ��ʽ��
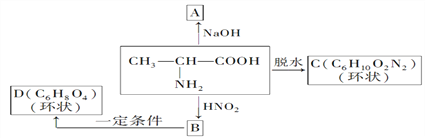
A______��B______��C______��D______��
(2)���������У�������NaOH��Ӧ�����������ᷴӦ������ˮ�����________(��ѡ���)��
��Al2O3����Al(OH)3���۰����ᡡ�ܶ��ġ�
��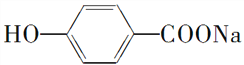 ����(NH4)2CO3
����(NH4)2CO3
��NaHCO3������ά�ء��ᵰ���ʡ���NH4I
A���٢ڢۢ� B���ܢݢޢߢ��
C���ۢܢݢޢ�� D��ȫ��
(3)�����йص����ʵ���������ȷ����________��
�ٵ�������Һ����뱥���������Һ���г����������ټ���ˮ��Ҳ���ܽ⣻
���¶�Խ�ߣ�ø���Ļ�ѧ��ӦԽ�죻
����Ȼ�����ʵ�ˮ�����ղ��X����Ϊ�������
���ؽ�������ʹ�����ʱ��ԣ�������ʳ�ؽ����λ��ж���
�ݰ�����͵����ʾ��Ǽ�����ǿ�ᷴӦ������ǿ�Ӧ���������ʣ�
��ͨ���þƾ���������Ϊ�ƾ���ʹϸ���еĵ����ʱ��Զ�ʧȥ�������ԣ�
����֯���Dz�˿��������˿��������������ζ�ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ոѣ����������ʣ����ۺ�Ӧ�þ�����Ҫ�����塣�������Խո�Ϊԭ�Ϻϳɾ�����߷��ӻ������·�ߣ�

�ش��������⣺
��1�����й��������˵����ȷ����______________�������ţ�
a.�������ζ������CnH2mOm��ͨʽ
b.��ѿ��ˮ�����ɻ�Ϊͬ���칹��������Ǻ���
c.��������Ӧ�����жϵ���ˮ���Ƿ���ȫ
d.���ۺ���ά�ض����ڶ�������Ȼ�߷��ӻ�����
��2��B����C�ķ�Ӧ����Ϊ______��
��3��D�й���������Ϊ______��D����E�ķ�Ӧ����Ϊ______��
��4��F �Ļ�ѧ������______����F����G�Ļ�ѧ����ʽΪ______��
��5������һ�ֹ����ŵĶ�ȡ�����㻯����W��E��ͬ���칹�壬0.5 mol W������̼��������Һ��Ӧ����44 gCO2��W����______�֣���������ṹ�������к˴Ź�������Ϊ�����Ľṹ��ʽΪ_________��
��6�����������ϳ�·�ߣ��ԣ���������-2��4-����ϩ��C2H4Ϊԭ�ϣ����Լ���ѡ��������Ʊ��Զ���������ĺϳ�·��_______________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com