
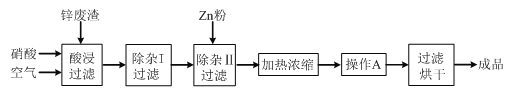
����Ŀ����ҵ�ϳ�����ұ��п�����е�п������ZnO��FeO��Fe2O3��CuO��Al2O3�����ʣ�������������Zn(NO3)2��6H2O���壬�乤������Ϊ��
�й��������↑ʼ�����ͳ�����ȫ��pH���±���
�������� | Al(OH)3 | Fe(OH)3 | Fe(OH)2 | Cu(OH)2 | Zn(OH)2 |
��ʼ������pH | 3��3 | 1��5 | 6��5 | 4��2 | 5��4 |
������ȫ��pH | 5��2 | 3��7 | 9��7 | 6��7 | 8��0 |
���ڡ�����������У�Ϊ���п�Ľ������ʣ���ͨ����������衱�⣬���ɲ�ȡ�Ĵ�ʩ��_____________________��
���������������жദ�漰�����ˡ���ʵ�����й��˲�����Ҫʹ�õIJ���������_____��
���ڡ�����I�������У����ټ�������H2O2��Һ��H2O2��Fe2+��Ӧ�����ӷ���ʽΪ_____��ΪʹFe(OH)3��Al(OH)3������ȫ����Zn(OH)2��������Ӧ������Һ��pH��ΧΪ_____������Fe3+�Ƿ������ȫ��ʵ�������_____��
�ȼ���Zn�۵�������_____��������A����������_____��
���𰸡��ʵ����߷�Ӧ�¶ȣ�����������Ũ�ȡ���п��������ȣ� �ձ�����������©�� 2Fe2++H2O2+2H+��2Fe3++2H2O 5.2��5.4 ���ã�ȡ�����ϲ���Һ���μ�KSCN��Һ���������ֺ�ɫ�������Fe3+������ȫ ��ȥ��Һ�е�Cu2+ ��ȴ�ᾧ
��������
��1��Ӱ�컯ѧ��Ӧ���ʵ�������Ũ�ȡ��¶ȡ���������Ĵ�С�ȣ������ڡ�����������У�Ϊ���п�Ľ������ʣ���ͨ����������衱�⣬���ɲ�ȡ�Ĵ�ʩ���ʵ����߷�Ӧ�¶ȣ�����������Ũ�ȡ���п��������ȣ���
��2����ʵ�����й��˲�����Ҫʹ�õIJ����������ձ�����������©����
��3���ڡ�����I�������У����ټ�������H2O2��Һ��H2O2�������ԣ���Fe2+�л�ԭ�ԣ�����������������ԭ��Ӧ�����ݵ����غ㡢����غ㼰ԭ���غ�ɵ�H2O2��Fe2+��Ӧ�����ӷ���ʽΪ2Fe2++H2O2+2H+��2Fe3++2H2O��ΪʹFe(OH)3��Al(OH)3������ȫ����Zn(OH)2���������������ʿ�ʼ�γɳ����ͳ�����ȫʱ��pH�Ĵ�С��Ӧ������Һ��pH��ΧΪ5.2��5.4������Fe3+�Ƿ������ȫ��ԭ����������Fe(SCN)3��Ѫ��ɫ��ʵ������Ǿ��ã�ȡ�����ϲ���Һ���μ�KSCN��Һ���������ֺ�ɫ�������Fe3+������ȫ��
��4��������Һ�л�����Cu2+��Ϊ����ȡ������Zn(NO3)2��6H2O���壬Ӧ�ð����ʳ��ӡ�����Zn�۵������dz�ȥ��Һ�е�Cu2+����Ӧ����ʽΪZn+Cu2+��Cu+Zn2+������Һ����Ũ������ȴ�ᾧȻ����˺�ɼ��õ�Zn(NO3)2��6H2O���塣


 ����ѵ����ֱͨ�п�����ϵ�д�
����ѵ����ֱͨ�п�����ϵ�д� һ���㶨ϵ�д�
һ���㶨ϵ�д� ��У��ҵ��ϵ�д�
��У��ҵ��ϵ�д� ���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ�ܱ������У�����1molN2��3molH2������һ���¶Ⱥ�ѹǿ�£�ʹ�䷢����Ӧ��N2(g)+3H2(g)![]() 2NH3(g) ��H= -92.4 kJ��mol-1���ش��������⣺
2NH3(g) ��H= -92.4 kJ��mol-1���ش��������⣺
(1)�����������ݻ����䣬�������г���1molN2����Ӧ���ʻ�________(�����ӿ�������������������������ͬ)��������____________��
(2)�����������ݻ����䣬�������г���1molHe����Ӧ���ʻ�________��������________��
(3)����������������ѹǿ���䣬�������г���1molHe����Ӧ���ʻ�________��������___________��
(4)�����������Ӧ���ʻ�________��������______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ѣ�Ti������Ϊ��������֮��ĵ����������Ѱף�TiO2����Ŀǰ��õİ�ɫ���ϡ��Ʊ�TiO2��Ti��ԭ�����������ҹ�������������������λ������Fe2O3����������Ҫ�ɷ�ΪFeTiO3����ȡTiO2���������£�
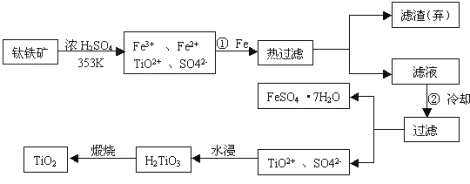
��1��Ti��ԭ������Ϊ22��λ��Ԫ�����ڱ��е�_________���ڵ�___________�塣
��2��������������Ŀ����___________________���������ȴ��Ŀ����__________��
��3��д������������H2TiO3�����ӷ���ʽ__________________________________��
��4�������Ʊ�TiO2�Ĺ����У����dzɱ��ͷ����ۺ��������أ���Һ��Ӧ����______������
��5���ɽ��ʯ(TiO2 )��ȡ����Ti���漰���IJ���Ϊ��TiO2��TiCl4![]() Ti
Ti
��֪���� C(s) + O2 (g) = CO2 (g) ��H = ��393.5 kJmol-1
�� 2CO(g) + O2 (g) = 2CO2(g) ��H =��566 kJmol-1
�� TiO2 (s) + 2Cl2 (g) = TiCl4 (s) + O2 (g) ��H = +141kJmol-1
��TiO2(s)+2Cl2(g)+2C(s)=TiCl4(s)+2CO(g) ����H =___________����ӦTiCl4+2Mg=2MgCl2+Ti��Ar�����н��е�������_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����û�ѧ��Ӧԭ��֪ʶ�о��������CO��SO2����Ⱦ������Ҫ���塣
��1����CO���Ժϳɼ״�����֪��
CH3OH(g)��3/2O2(g)===CO2(g)��2H2O(l) ��H����764.5 kJ��mol��1
CO(g)��1/2O2(g)===CO2(g) ��H����283.0 kJ��mol��1
H2(g)��1/2O2(g)===H2O(l) ��H����285.8 kJ��mol��1
��CO(g)��2H2(g) ![]() CH3OH(g) ��H��______kJ��mol��1��
CH3OH(g) ��H��______kJ��mol��1��
��2�����д�ʩ���ܹ����������ϳɼ״���Ӧ���ʵ���________(��д���)��
a��ʹ�ø�Ч���� b�����ͷ�Ӧ�¶�
c��������ϵѹǿ d�����Ͻ�CH3OH�ӷ�Ӧ������з������
��3����һ��ѹǿ�£��ݻ�ΪV L�������г���a mol CO��2a mol H2���ڴ��������·�Ӧ���ɼ״���ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ
����ͼ��ʾ��
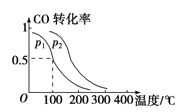
��p1________p2(����ڡ�����С�ڡ����ڡ�)��
��100 ��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K��________��
���������������������£�������a mol CO��2a mol H2���ﵽ��ƽ��ʱ��CO��ת����________(���������С�����䡱)��
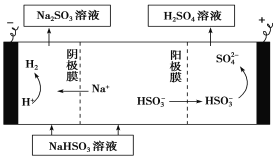
��4��ij����С����SO2Ϊԭ����ȡ���ᡣ
������ԭ���ԭ������SO2��O2��H2O���Ʊ����ᣬ�õ���ö�ײ������缫�������������壬ͬʱҲ��ʹ������������Һ��ֽӴ�����д���õ�ظ����ĵ缫��Ӧʽ��_____________________��
����Na2SO3��Һ�������SO2��NaHSO3��Һ��Ȼ�������Һ���Ƶ����ᡣ���ԭ��ʾ��ͼ���¡���д����ʼʱ������Ӧ�ĵ缫��Ӧʽ��_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���й����ʵ����ļ�����գ�
��1��2 mol����[CO(NH2)2]��_____________��Hԭ�ӣ�������ԭ�Ӹ�__________g H2O������ԭ�Ӹ�����ȡ�
��2���ٱ�״���£�22.4 L CH4����1.5 mol NH3����1.806��1024��H2O���ܱ�״���£�73 g HCl������Hԭ�Ӹ����ɶൽ�ٵ�˳����____________________��
��3��30.9 g NaR����Na��0.3 mol����NaR��Ħ������Ϊ_________________��
��4��100mLijAl2(SO4)3��Һ�У�c(Al3��)��2.0 mol��L��1��������c(SO42��)��_________mol��L��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����Ȼ��ƺ������Ϊԭ���Ʊ��Ȼ�識�����Ʒ������,������������:
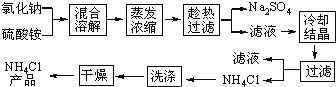
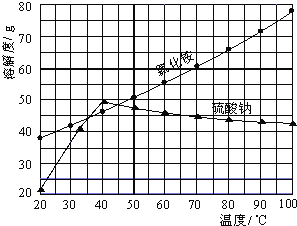
�Ȼ�狀������Ƶ��ܽ�����¶ȱ仯����ͼ��ʾ���ش��������⣺
��1�����Ʊ�10.7gNH4Cl����������NaCl_________g��
��2��ʵ���ҽ�������Ũ���õ�����Ҫ������________���ձ������������ƾ��Ƶȡ�
��3������ȴ�ᾧ�������У�����NH4Cl����ĺ����¶�Ϊ_________��
��4�����������Լ������NH4Cl��Ʒ�Ƿ��ķ�����������_________________��
��5����NH4Cl��Ʒ�к������������ʣ���һ���ᴿ��Ʒ�ķ�����________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��������ʵ��װ��һ������ʲôʵ���������ʵ��������ƣ�
A __________ B ___________ C ___________
A��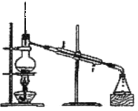 B��
B��![]() C��
C��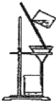
����3p%��������ͬ�����p%�������ϵõ�q%��ϡ���ᣬ��p��q�Ĺ�ϵ��ȷ����______��
��2����ͬ�¡�ͬѹ�£�ʵ����CO��N2��SO2��������Ļ��������ܶ���H2��18.5��������SO2����������Ϊ ________��������CO��N2�����ʵ���֮��Ϊ1��1��������������Ԫ�ص���������Ϊ ________����С�������1λ��
��3����ͬ�����£�ijCl2��O2�������200 mLǡ����300 mL H2��������HCl��H2O����֪��H2+Cl2=2HCl��������������Cl2��O2�������Ϊ_____����������ƽ��Ħ������Ϊ_______________����С�������1λ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±������������г��������ʣ������г������ǵģ���Ҫ���ɷ�
��� | �� | �� | �� | �� | �� | �� | �� |
���� | �ƾ� | ���� | ��� | ʳ�� | ͭ���� | �������� | �մ� |
��Ҫ�ɷ� | CH3CH2OH | CH3COOH | NaOH | NaCl | Cu | SO2 | Na2CO3 |
��1������Ա��Т�~�ߵ���Ҫ�ɷֽ��з��ࣨ���ţ���
���ڵ���ʵ���_______________ �����ڷǵ���ʵ���____________________ ��
��2�������ڵ�ˮ��Һ��߷�Ӧ�����ӷ���ʽ ________________________________��
��3����֪�ý�������ȡ�����ƣ����ж��ַ�����
��4Na + O2=2Na2O�� ��4Na + CO2 =2Na2O + C����2NaNO2 + 6Na =4Na2O + N2��
���������ַ����У���õ���_________ ��ԭ����___________��
��4��ijʵ��С��ͨ������ʵ��̽������������ˮ�ķ�Ӧ��

���û�ѧ����ʽ����ʹ��̪����ԭ�� _______________________________������ʵ�������Ʋ��ɫ��ȥ��ԭ����___________________________________��
�ڼ���MnO2��Ӧ�Ļ�ѧ����ʽΪ ___________________________________ ��
��5����ʵ����ģ������Ƽ�Ʊ�̼���ƣ�һ���¶��£���һ��������NaCl��Һ��ͨ�백���ﵽ���ͺ��ٲ���ͨ��CO2��һ��ʱ����ֳ��������˵õ�NaHCO3���塣�ù��̵Ļ�ѧ����ʽΪ��____________
��6���ڻ�ѧ������ʦ��NaOH��Һͨ��CO2���������������������ͨ��ʵ��֤��CO2��NaOH�����˷�Ӧ��ij��ѧС��ͬѧ�������ĸɱ���������������Һ�У��������ֻ��������ⶨ��ҺpH�ı仯����ͼ��ʾ�����û�ѧ����ʽ�ش��������⣺
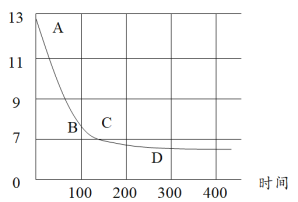
��BC�α仯ԭ������� ___________________________________________________________ ��
��CD�α仯ԭ������� ___________________________________________________________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ԫ��R��T��Q��W��Ԫ�����ڱ��е����λ����ͼ��ʾ������T������������������������ȡ������жϲ���ȷ���ǣ� ��
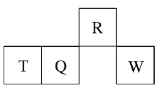
A. �����̬�⻯������ȶ��ԣ�R��Q
B. ����������Ӧˮ��������ԣ�Q��W
C. ԭ�Ӱ뾶��T��Q��R
D. ����T��NaOH��Һ����Ӧ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com