
����Ŀ��CO��H2����Ϊ��Դ�ͻ���ԭ�ϣ�Ӧ��ʮ�ֹ㷺����ҵ�Ͽ�����CO��CO2��H2��Ӧ���Ʊ��״���
��Ӧ�٣�2H2(g)+CO(g)![]() CH3OH(g)��H����90.8kJ��mol-1
CH3OH(g)��H����90.8kJ��mol-1
��Ӧ�ڣ�H2(g)+CO2(g)![]() H2O(g)+CO(g)��H��+41.2kJ��mol-1
H2O(g)+CO(g)��H��+41.2kJ��mol-1
��1��д����CO2��H2��Ӧ�Ʊ��״����Ȼ�ѧ����ʽ______________��
��2����֪�������һ����̼��ˮ�������뷴Ӧ��ʱ���ᷢ�����·�Ӧ��CO(g)+H2O(g)![]() H2(g)+CO2(g)���÷�Ӧƽ�ⳣ�����¶ȵı仯�����ʾ��
H2(g)+CO2(g)���÷�Ӧƽ�ⳣ�����¶ȵı仯�����ʾ��
�¶�/�� | 400 | 500 | 800 |
ƽ�ⳣ��K | 9.94 | 9 | 1 |
�����¶ȣ���ƽ����ƶ�������______________�������������500��ʱ��CO��H2O����ʼŨ�Ⱦ�Ϊ0.020mol��L-1����������CO��ƽ��Ũ��Ϊ��______________mol��L-1��
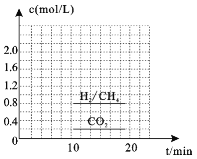
��3��һ�������£�������CO2(g)��H2(g)�ϳ�CH4(g)��ͬʱ������H2O(g)��������ܱ������г���һ������CO2��H2����300��ʱ����������Ӧ��10min�ﵽƽ��ʱ�������ʵ���Ũ����ͼ��ʾ,���¶��µĵ�ƽ�ⳣ������_______________��
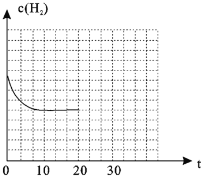
��4������20minʱ��Сѹǿ������30minʱ�ﵽƽ��״̬������ͼ2�л���H2�����ʵ���Ũ����ʱ��仯��ͼ��__________________��
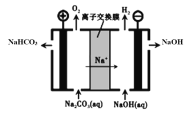
��5����ҵ�ϵ��Na2CO3��Һ��������NaHCO3��NaOH���ֹ�ҵ��Ҫԭ�ϣ�װ����ͼ��ʾ����д�������ĵ缫��Ӧʽ______________________��
���𰸡�CO2(g)+3H2(g)��CH3OH(g)+H2O(g)��H=-49.6kJ��mol-1 ���� 0.005 25 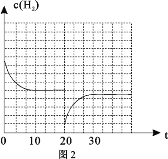 4CO32-+2H2O-4e-��4HCO3-+O2��
4CO32-+2H2O-4e-��4HCO3-+O2��
��������
��1����֪����Ӧ�٣�2H2(g)+CO(g)![]() CH3OH(g)��H����90.8kJ��mol-1
CH3OH(g)��H����90.8kJ��mol-1
��Ӧ�ڣ�H2(g)+CO2(g)![]() H2O(g)+CO(g)��H��+41.2kJ��mol-1
H2O(g)+CO(g)��H��+41.2kJ��mol-1
�ɸ�˹���ɿ�֪��+�ڿɵ�CO2(g)+3H2(g)��CH3OH(g)+H2O(g)��H=-49.6kJ��mol-1���ʴ�Ϊ��CO2(g)+3H2(g)��CH3OH(g)+H2O(g)��H=-49.6kJ��mol-1��
��2���¶�����Kֵ��С����ƽ�����淽���ƶ�����CO��Ũ�ȱ仯Ϊxmol/L��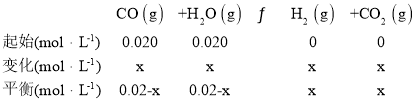
![]() ��x=0.015mol/L����CO��ƽ��Ũ��Ϊ0.02 mol/L -0.015 mol/L=0.005 mol/L���ʴ�Ϊ������0.005��
��x=0.015mol/L����CO��ƽ��Ũ��Ϊ0.02 mol/L -0.015 mol/L=0.005 mol/L���ʴ�Ϊ������0.005��
��3��һ�������£�������CO2(g)��H2(g)�ϳ�CH4(g)��ͬʱ������H2O(g)��ӦΪ��![]() ������ͼ���֪c(CH4)=0.8mol/L����c(H2O)= 2c(CH4)=1.6mol/L��
������ͼ���֪c(CH4)=0.8mol/L����c(H2O)= 2c(CH4)=1.6mol/L��  ���ʴ�Ϊ��25��
���ʴ�Ϊ��25��
��4��![]() ��Сѹǿ�������������Ũ�ȼ�С��ƽ�����ƣ�������������ԭ����֪����Ũ��С��ƽ��10min��ʱŨ�ȣ�ͼ��Ϊ
��Сѹǿ�������������Ũ�ȼ�С��ƽ�����ƣ�������������ԭ����֪����Ũ��С��ƽ��10min��ʱŨ�ȣ�ͼ��Ϊ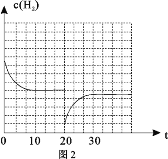 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��5���ɵ��װ��ʾ��ͼ��֪���͵�Դ����������Ϊ�����������ΪNa2CO3��Һ����O2��NaHCO3�������缫��ӦΪ��4CO32-+2H2O-4e-��4HCO3-+O2�����ʴ�Ϊ��4CO32-+2H2O-4e-��4HCO3-+


 �������ͬ����ϰϵ�д�
�������ͬ����ϰϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��Ӧ�з�Ӧ�����������У�AsH3��H2SO4��KBrO3��K2SO4��H3AsO4��H2O��һ��δ֪����X��
��1����֪KBrO3�ڷ�Ӧ�еõ����ӣ�����_____�����������ԭ������Ӧ����÷�Ӧ�Ļ�ԭ����________��
��2����֪0.2molKBrO3�ڷ�Ӧ�еõ�1mol��������X����X�Ļ�ѧʽΪ____��
��3������������Ӧ����֪________(�����)��
a�������ԣ�KBrO3>H3AsO4
b�������ԣ�H3AsO4>HBrO3
c����ԭ�ԣ�AsH3>X
d����ԭ�ԣ�X>AsH3
��4�����������ͻ�ԭ���Ļ�ѧʽ������ƽ���ϵ���������з����У����������ת�Ƶķ������Ŀ��_____________
![]() ��
��![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ˮ��ɽ���ǽ�ɽ��ɽ��������������ɫ��չ����̬������Ϊ�й�չʾ�������һ��������Ƭ����
(I)����β������ɴ�����Ⱦ����Ҫԭ��֮һ�����ٵ����������ڴ����е��ŷ��ǻ�����������Ҫ����֮һ����ش��������⣺
(1)��֪��![]()
![]()
![]()
![]()
��ij��Ӧ��ƽ�ⳣ������ʽΪ![]() ����˷�Ӧ���Ȼ�ѧ����ʽΪ_______��
����˷�Ӧ���Ȼ�ѧ����ʽΪ_______��
(2)![]() ��һ�������¿ɷ����ֽ⣺
��һ�������¿ɷ����ֽ⣺![]() ��һ���¶��£��ں����ܱ������г���һ����
��һ���¶��£��ں����ܱ������г���һ����![]() ���и÷�Ӧ�����жϷ�Ӧ�Ѵﵽ��ѧƽ��״̬����_____(����ĸ)��
���и÷�Ӧ�����жϷ�Ӧ�Ѵﵽ��ѧƽ��״̬����_____(����ĸ)��
a. ![]() ��
��![]() ��Ũ�ȱȱ��ֲ��� b.������ѹǿ���ٱ仯
��Ũ�ȱȱ��ֲ��� b.������ѹǿ���ٱ仯
c. ![]() d. ������ܶȱ��ֲ���
d. ������ܶȱ��ֲ���
(��)�״����Ҵ���Դ�ḻ���۸�������������淽�㣬������Ҫ�Ļ���ԭ�ϣ�������Ҫ����;��Ӧ��ǰ���������ö��ַ����ϳɡ� CO2(g) + 3H2(g)![]() CH3OH(g) + H2O(g)
CH3OH(g) + H2O(g)
(3)��![]() ��
��![]() �����ʵ���֮��1��3�������Ϊ2.0L�ĺ����ܱ������з�Ӧ����
�����ʵ���֮��1��3�������Ϊ2.0L�ĺ����ܱ������з�Ӧ����![]() ����ͼ1��ʾѹǿΪ0.1 MPa��5.0 MPa��
����ͼ1��ʾѹǿΪ0.1 MPa��5.0 MPa��![]() ת�������¶ȵı仯��ϵ��
ת�������¶ȵı仯��ϵ��
��a��b���㻯ѧ��Ӧ���ʷֱ���![]() ����ʾ����
����ʾ����![]() _____
_____![]() (����������������������������)
(����������������������������)
���г�a���Ӧ��ƽ�ⳣ������ʽK= ____________________��
(4)��1.0 L�����ܱ�������Ͷ��1 mol ![]() ��2.75 mol
��2.75 mol ![]() ������Ӧ��
������Ӧ��![]() CH3OH(g) + H2O(g)��ʵ���ò�ͬ�¶ȼ�ѹǿ�£�ƽ��ʱ�״������ʵ����仯��ͼ2��ʾ������˵����ȷ����________��
CH3OH(g) + H2O(g)��ʵ���ò�ͬ�¶ȼ�ѹǿ�£�ƽ��ʱ�״������ʵ����仯��ͼ2��ʾ������˵����ȷ����________��
A.�÷�Ӧ������ӦΪ���ȷ�Ӧ
B.ѹǿ��С��ϵΪP1��P2��P3
C.M���Ӧ��ƽ�ⳣ��K��ֵԼΪ![]()
D.��![]() ��512 Kʱ��ͼ��N��
��512 Kʱ��ͼ��N��![]()
(5)![]() ������ϳ��Ҵ��ķ�ӦΪ��2CO2(g) + 6H2(g)
������ϳ��Ҵ��ķ�ӦΪ��2CO2(g) + 6H2(g) ![]() C2H5OH(g) + 3H2O(g)
C2H5OH(g) + 3H2O(g) ![]() ��m������ʼʱ��Ͷ�ϱȣ���
��m������ʼʱ��Ͷ�ϱȣ���![]() ��
��
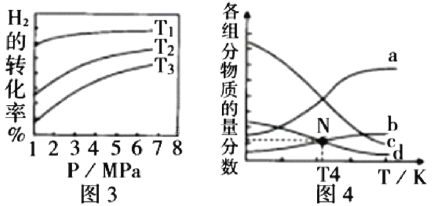
�� ͼ3��Ͷ�ϱ���ͬ���¶�![]() ����÷�Ӧ���ʱ�
����÷�Ӧ���ʱ�![]() _______0(��
_______0(��![]() )��
)��
�� m=3ʱ����ѹ�����¸����ʵ����ʵ����������¶ȵĹ�ϵ��ͼ4��ʾ��������b����������Ϊ_________(�ѧʽ)��
(6)�Լ״�Ϊ��Ҫԭ�ϣ��绯ѧ�ϳ�̼��������Ĺ���ԭ����ͼ5��ʾ�����ӽ���ĤaΪ ______(������Ĥ��������Ĥ��)�������ĵ缫��ӦʽΪ_______________________________________

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������X����(��ͨ��)Y��Һ�У������ɳ��������ʵ��������X�����ʵ����Ĺ�ϵ����ͼ��ʾ������ͼʾ�������

A.AB.BC.CD.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ�����Ʊ��������Ҫ������������ʾ��

�������ڲ�ͬ�¶��µ��ܽ�ȣ�g/100gH2O����
�¶� ���� | 0�� | 10�� | 20�� | 30�� | 40�� | 50�� | 60�� | 100�� |
NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 | 39.8 |
NH4HCO3 | 11.9 | 15.8 | 21.0 | 27.0 | - | - | - | - |
NaHCO3 | 6.9 | 8.1 | 9.6 | 11.1 | 12.7 | 14.5 | 16.4 | - |
NH4Cl | 29.4 | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 | 55.3 | 77.3 |
��ʾ���¶ȸ���35��ʱNH4HCO3��ֽ⣬��ش�
��1�����в�����������ȷ����________��
A���¶ȿ�����30-35������Ϊ�¶�̫��NH4HCO3��ֽ⣬�¶�̫�ͷ�Ӧ����̫��
B������30min��Ŀ����ʹ��Ӧ��ֽ���
C�����˺����Һֻ��NH4Cl��NH4HCO3����
D��ϴȥ�����������ʿ���ѡ������ˮ
��2����Ӧ�¶ȿ�����30��35�棬Ϊ���ƴ��¶ȷ�Χ����ȡ�ļ��ȷ���Ϊ______________��
��3������ʱ�����˺���Ҫ�õ�NaHCO3�����ԭ����______________��
��4������NaHCO3�����װ��Ϊ________��
A. B.
B. C.
C.
��5��ϴ��NaHCO3����IJ���______________��
��6���ⶨ�����Ʒ��NaHCO3�����ķ�����ȷ��ȡ������ƷWg������ƿ�м�����ˮ�ܽ⣬��1��2�η�ָ̪ʾ���������ʵ���Ũ��Ϊc��mol��L-1����HCl��Һ�ζ�����Һ�ɺ�ɫ����ɫ(ָʾCO32-+H+=HCO3����Ӧ���յ�)����HCl��Һ���ΪV1mL���ټ�1��2�μ���ָʾ����������HCl��Һ�ζ�����Һ�ɻƱ�ȣ�����HCl��Һ���ΪV2mL��д��������Ʒ��NaHCO3���������ļ���ʽ��______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����20mL��0.2mol/LNaOH��Һ�У�����ͨ��һ����CO2���壬����Һ��һ�����������ɡ�
��1�����õ���ɫ�����Ǵ������ͨ���CO2�����Ϊ__mL��__mL���������д���йص����ӷ���ʽ��__��__��
��2����������ɫ�����ˮ�ܽ⣬��������pH��7�����ɵð�ɫ��������Ϊ__g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ʢ��KL��Һ���Թ��м�������![]() ��μ���ˮ��
��μ���ˮ��![]() ������ɫ������������Թ��еμ���ˮ����
������ɫ������������Թ��еμ���ˮ����![]() �����dz���������ɫ��
�����dz���������ɫ��
���������գ�
1) д������ƽ![]() ������ɫ�����ɫ�Ļ�ѧ��Ӧ����ʽ(���ϵ����1��������д)��
������ɫ�����ɫ�Ļ�ѧ��Ӧ����ʽ(���ϵ����1��������д)��
 _____
_____
2)���������еĻ�ԭ����___��
3)��KL����KBr����![]() ���Ϊ__ɫ�������μ���ˮ��
���Ϊ__ɫ�������μ���ˮ��![]() �����ɫû�б仯��
�����ɫû�б仯��![]() ��
��![]() ��
��![]() ��������ǿ������˳����______��
��������ǿ������˳����______��
4)�ӵ����к�����Ϊ20mg��50mg��kg����ȡ�ӵ���(��![]() ��ʳ��)1000kg����ׯKl��
��ʳ��)1000kg����ׯKl��![]() ��Ӧ��
��Ӧ��![]() ��������Ҫ����
��������Ҫ����![]() ________L(��״��������2λС��)��
________L(��״��������2λС��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ӷ���ʽ��ȷ���� ��������
A. ��Ba(OH)2��Һ�еμ�NaHSO4��Һ�������պ���ȫ��Ba2+ + 2OH�� + 2H+ + SO42�� = BaSO4��+ 2 H2O
B. AlCl3��Һ�м��������ˮ��Al3+��4OH��=== ![]() ��2H2O
��2H2O
C. ��̼�������Һ�м����������������Ca2����2HCO3����2OH����CaCO3����2H2O��CO32��
D. ����ʯ��ˮ��̼������Һ��Ӧ��Ca(OH)2+CO32-=CaCO3��+OH-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��NH3����Ҫ�Ļ���ԭ�ϣ���һϵ�з�Ӧ�ɵõ�HNO3��CO(NH2)2��HCN�Ȳ�Ʒ��
(1)����Ϊԭ�Ϻϳ�HNO3�������£�д��N2O4ת��ΪHNO3�ķ�Ӧ����ʽ_____��
![]()
(2)��NH3��CO2Ϊԭ���������صķ�Ӧ���£�
��Ӧ��2NH3(l) �� CO2(g) ![]() NH2COONH4(l) �� H1 �� a kJ��mol-1
NH2COONH4(l) �� H1 �� a kJ��mol-1
��Ӧ����NH2COONH4(l) ![]() NH2CONH2(l) �� H2O(l) �� H2 �� b kJ��mol-1
NH2CONH2(l) �� H2O(l) �� H2 �� b kJ��mol-1
�� ��֪NH3(l) ![]() NH3(g) �� H3 �� c kJ��mol-1 ��Ӧ2NH3(g) �� CO2(g)
NH3(g) �� H3 �� c kJ��mol-1 ��Ӧ2NH3(g) �� CO2(g) ![]() NH2CONH2(l) �� H2O(l) �� H4��_____kJ��mol-1(�ú�a��b��c��ʽ�ӱ�ʾ)���÷�Ӧ���Է����е���Ҫԭ����_____��
NH2CONH2(l) �� H2O(l) �� H4��_____kJ��mol-1(�ú�a��b��c��ʽ�ӱ�ʾ)���÷�Ӧ���Է����е���Ҫԭ����_____��
�� һ�������£��������ܱ�������Ͷ��4 mol NH3�� 1 mol CO2����ø�������ʵ�����ʱ��仯��ͼ1������˵����ȷ����_____��
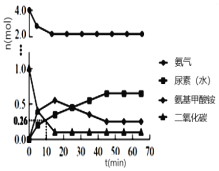
A��ѡ����ʵ�̼������������߲���
B����Ӧ��Ļ�ܱȷ�Ӧ��С
C���������������뷴Ӧ���ɵ�ˮ��ϣ����������غϳ�
D���������ʵ������ٱ仯ʱ����Ӧ�ﵽƽ��״̬
(3)��ҵ�����ð�������������ķ�ӦΪCH4(g) + NH3(g) ![]() HCN(g) + 3H2(g) �� H>0
HCN(g) + 3H2(g) �� H>0
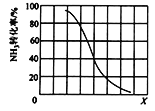
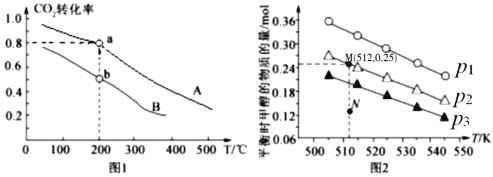
�� ��������һ�����ﵽƽ��ʱNH3ת�������������X�仯�Ĺ�ϵ��ͼ��ʾ��X��������_____(�����¶�������ѹǿ��)��

�� ��������һ��ʱ����2 L�ܱ������м���n mol CH4��2 mol NH3��ƽ��ʱNH3���������n�仯�Ĺ�ϵ��ͼ��ʾ��ƽ�ⳣ��K=_____����д��������̣�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com