
A��B��D��E��G��M�������ֳ���Ԫ�أ����ǵĺ˵���������������У�Ԫ��M�Ļ�̬3d�������2�����ӣ�A�Ļ�̬ԭ��L���������K���������2����E�ļ�������ͬ����Ԫ�صļ������а뾶��С��D��Gͬ���壻B��D�γɵĻ������ж��֣�����һ���Ǻ���ɫ���塣
(1)M�Ļ�̬ԭ�Ӽ۲�����Ų�ʽΪ___________________________________________��Ԫ��B��D��G�ĵ�һ�������ɴ�С��˳����________________________(��Ԫ�ط��ű�ʾ)��
(2)�ü۲���ӶԻ�������Ԥ�⣬GD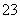 �����幹����____________(�����ֱ���)��
�����幹����____________(�����ֱ���)��
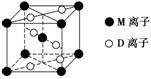
(3)M��D�γɵ�һ�ֳȺ�ɫ���徧���ṹ����ͼ��ʾ���仯ѧʽΪ________(��Ԫ�ط��ű�ʾ)��
(4)��֪������EB�ṹ�뵥�������ƣ������ʿ���E���Ȼ�����NaB3�ڸ����·�Ӧ�Ƶã������ɵ���B2���÷�Ӧ��ѧ����ʽΪ_______________________________________________������8.4 g B2���ɣ���ת�Ƶ�����Ϊ________��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ԫ�����ڱ��еڢ�A��Ԫ�صĵ��ʼ��仯�������;�㷺��
(1)����Ԫ��ͬ��Ķ�����Ԫ�ص�ԭ�ӽṹʾ��ͼΪ________��
(2)����Ϊ�ȡ��塢��Ԫ�طǽ�����(ԭ�ӵõ�������)�ݱ���ɵ��ж�������________(�����)��
a��Cl2��Br2��I2���۵�
b��Cl2��Br2��I2��������
c��HCl��HBr��HI�����ȶ���
d��HCl��HBr��HI������
(3)��ҵ�ϣ�ͨ������ת�����Ƶ�KClO3���壺
NaCl��Һ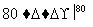 NaClO3��Һ
NaClO3��Һ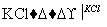 KClO3�������ɢ��з�Ӧ���ܻ�ѧ����ʽ��
KClO3�������ɢ��з�Ӧ���ܻ�ѧ����ʽ��
 NaCl��
NaCl�� H2O===
H2O=== NaClO3��
NaClO3�� ________��
________��
�ڢ���ת���Ļ�����Ӧ������________________���÷�Ӧ����������KClO3���������������������ԭ����____________________________________��
(4)һ�������£���ˮ��Һ��1 mol Cl����ClO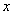 (x��1��2��3��4)������(kJ)��Դ�С����ͼ��ʾ��
(x��1��2��3��4)������(kJ)��Դ�С����ͼ��ʾ��
��D��________(�����ӷ���)��
��B��A��C��Ӧ���Ȼ�ѧ����ʽΪ________________(�����ӷ��ű�ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����йصζ�������˳����ȷ����(����)
���ñ���Һ��ϴ�ζ��� �����ζ�����ע�����Һ
�ۼ��ζ����Ƿ�©ˮ �ܵζ��� ��ϴ��
A���ݢ٢ڢۢ� B���ۢݢ٢ڢ� C���ۢ٢ݢڢ� D���ݢ٢ۢڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��t���£�ij��Ӧ�ﵽƽ�⣬ƽ�ⳣ��K��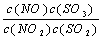 ������ʱ���¶����ߣ�NOŨ�ȼ�С������˵����ȷ���� ( )
������ʱ���¶����ߣ�NOŨ�ȼ�С������˵����ȷ���� ( )
A���÷�Ӧ���ʱ�Ϊ��ֵ
B��K����ʽ�У�C��SO3��ָ��Ӧ�ڸ������´ﵽ��ѧƽ��ʱSO3�����ʵ���Ũ��
C�������¶ȣ��淴Ӧ���ʼ�С
D���÷�Ӧ��ѧ����ʽΪ��NO��SO3 NO2��SO2
NO2��SO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
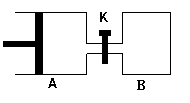
��ͼ��ʾ������A����ǿ�ƽ���ƶ��Ļ�������A�г���1molX��1molY����B�г���2molX��2molY����ʼʱV(A)=V(B)=aL������ͬ�¶Ⱥ��д������ڵ������£����������Է�����X(g)+Y(g)  Z(g)+2W(g)��
Z(g)+2W(g)��
�ﵽƽ��ʱV(A)=1.2aL���Իش�
��A��X��ת����Ϊ ��
��A��B��Xת���ʴ�С��ϵΪA B�������������������=�� ��
�Ǵ�K��һ��ʱ����ִﵽ�µ�ƽ��ʱ��A�����Ϊ L����ͨ��������������ƣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�±����������Ȼ�����۵�ͷе㣺
| NaCl | MgCl2 | AlCl3 | SiCl4 | |
| �۵�/�� | 801 | 714 | 190 | ��70 |
| �е�/�� | 1 413 | 1 412 | 180 | 57.57 |
�йر������������Ȼ�������ʣ����������������Ȼ����ڼ���ʱ�������������Ȼ����ھ�̬ʱ���ڷ��Ӿ��壬���Ȼ��ƾ�������֮���Է��»�����ϣ����Ȼ��������ǵ��͵����Ӿ��塣�������������һ�µ���
A���٢ڡ��������������� B���ڢ�
C���٢ڢ� D���ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��H2O2��H2SO4�Ļ����Һ���ܳ��Ͼ�ӡˢ��·���ϵ�ͭ����֪��
Cu(s)��2H��(aq)===Cu2��(aq)��H2(g)
��H����64.39 kJ��mol��1
2H2O2(l)===2H2O(l)��O2(g)
��H����196.46 kJ��mol��1
H2(g)�� O2(g)===H2O(l)
O2(g)===H2O(l)
��H����285.84 kJ��mol��1
��H2SO4��Һ�У�Cu��H2O2��Ӧ����Cu2��(aq)��H2O(l)�ķ�Ӧ�Ȧ�H����
A����417.91 kJ��mol��1���� B����319.68 kJ��mol��1
C����546.69 kJ��mol��1  D����448.46 kJ��mol��1
D����448.46 kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪Fe3O4��1 mol Al��Ӧת��ΪFe����ʱ�ų�a kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ___________________________________________________��
(2)(2012������)���÷�Ӧ��4HCl��O2 2Cl2��2H2O����ʵ���ȵ�ѭ�����á�
2Cl2��2H2O����ʵ���ȵ�ѭ�����á�
��֪��
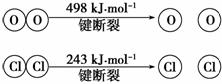
��������Ӧ�У�4 mol HCl���������ų�115.6 kJ����������÷�Ӧ���Ȼ�ѧ����ʽ��_________________________________________________________________ _______��
_______��
�Ͽ�1 mol H��O����Ͽ�1 mol H��Cl�������������ԼΪ________ kJ��
(3) (2012���¿α�ȫ��)��ҵ��������Ȼ��(��Ҫ�ɷ�ΪCH4)��CO2���и��������Ʊ�CO����֪CH4��H2��CO��ȼ����(��H)�ֱ�Ϊ��890.3 kJ��mol��1����285.8 kJ��mol��1�ͣ�283.0 kJ��mol��1��������1 m3(��״��)CO��������Ϊ________��
(2012���¿α�ȫ��)��ҵ��������Ȼ��(��Ҫ�ɷ�ΪCH4)��CO2���и��������Ʊ�CO����֪CH4��H2��CO��ȼ����(��H)�ֱ�Ϊ��890.3 kJ��mol��1����285.8 kJ��mol��1�ͣ�283.0 kJ��mol��1��������1 m3(��״��)CO��������Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
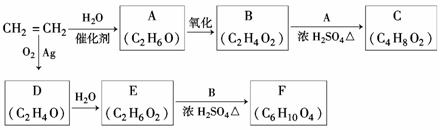
��ϩ��һ����Ҫ�Ļ���ԭ�ϣ�����ϩΪԭ�����������ֻ�����Ʒ�ķ�Ӧ����(���ַ�Ӧ��������ȥ)��
��ش��������⣺
(1)A�Ļ�ѧ������_______________________________________________��
(2)B��A��Ӧ����C�Ļ�ѧ����ʽΪ______________________________���÷�Ӧ������Ϊ_ ___________________________________________________��
___________________________________________________��
(3)D�Ľṹ��ʽΪ_________________________________________________________��
(4)F�Ľṹ��ʽΪ________________________________________________��
(5)D��ͬ���칹��Ľṹ��ʽΪ_____________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com