
�±����������Ȼ�����۵�ͷе㣺
| NaCl | MgCl2 | AlCl3 | SiCl4 | |
| �۵�/�� | 801 | 714 | 190 | ��70 |
| �е�/�� | 1 413 | 1 412 | 180 | 57.57 |
�йر������������Ȼ�������ʣ����������������Ȼ����ڼ���ʱ�������������Ȼ����ھ�̬ʱ���ڷ��Ӿ��壬���Ȼ��ƾ�������֮���Է��»�����ϣ����Ȼ��������ǵ��͵����Ӿ��塣�������������һ�µ���
A���٢ڡ��������������� B���ڢ�
C���٢ڢ� D���ڢ�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������H��He��N��Na��Mg��Si��Ԫ�أ�������δ������Դ���⡣
(1)3He�Ǹ�Ч����ԭ�ϣ���ԭ�Ӻ���������Ϊ________��
(2)Na��ԭ�ӽṹʾ��ͼΪ________��Na����������ȫȼ�����ò���ĵ���ʽΪ________��
(3)MgCl�ڹ�ҵ��Ӧ�ù㷺������MgO�Ʊ���
��MgO���۵��BaO���۵�________(��ߡ��͡�)��
��������ij��ʯ�������õ���MgO�к�������SiO2����ȥSiO2�����ӷ���ʽΪ__________________________��SiO2�ľ�������Ϊ________��
��MgO��̿�ۺ�������һ�������·�Ӧ���Ʊ�MgCl2����β����������NaOH��Һ��ȫ���գ������ɵ���Ϊ________________(д��ѧʽ)��
(4)�����к��зḻ��3He��������������1 kg 3He��ͬʱ�ɵ�6000 kg H2��700 kg N2����������H2��N2Ϊԭ�Ͼ�һϵ�з�Ӧ��������̼�����________kg��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��25��������£��������Ϊ10 mL��pH������3�Ĵ�������ᣬ��ˮϡ�͵�a mL��b mL�����ϡ�ͺ���Һ��pH��Ϊ5����ϡ��ʱ����ˮ�����(����)��
A��a��b��10 mL B��a��b>10 mL
C��a<b D��a>b
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
X��Y��Z��M��G����Ԫ�ط�������������,��ԭ��������������X��Zͬ����,���γ����ӻ�����ZX;Y��Mͬ����,���γ�MY2��MY3���ַ��ӡ�
��ش���������:
(1)Y��Ԫ�����ڱ��е�λ��Ϊ�� ��
(2)����Ԫ�ص�����������Ӧ��ˮ����������ǿ������������(д��ѧʽ),�ǽ�����̬�⻯�ﻹԭ����ǿ������������(д��ѧʽ)��
(3)Y��G�ĵ��ʻ���Ԫ��֮���γɵĻ��������ˮ����������______________
(д�������������ʵĻ�ѧʽ)��
(4)ZX�ĵ���ʽΪ�� ��[
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
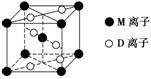
A��B��D��E��G��M�������ֳ���Ԫ�أ����ǵĺ˵���������������У�Ԫ��M�Ļ�̬3d�������2�����ӣ�A�Ļ�̬ԭ��L���������K���������2����E�ļ�������ͬ����Ԫ�صļ������а뾶��С��D��Gͬ���壻B��D�γɵĻ������ж��֣�����һ���Ǻ���ɫ���塣
(1)M�Ļ�̬ԭ�Ӽ۲�����Ų�ʽΪ___________________________________________��Ԫ��B��D��G�ĵ�һ�������ɴ�С��˳����________________________(��Ԫ�ط��ű�ʾ)��
(2)�ü۲���ӶԻ�������Ԥ�⣬GD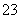 �����幹����____________(�����ֱ���)��
�����幹����____________(�����ֱ���)��
(3)M��D�γɵ�һ�ֳȺ�ɫ���徧���ṹ����ͼ��ʾ���仯ѧʽΪ________(��Ԫ�ط��ű�ʾ)��
(4)��֪������EB�ṹ�뵥�������ƣ������ʿ���E���Ȼ�����NaB3�ڸ����·�Ӧ�Ƶã������ɵ���B2���÷�Ӧ��ѧ����ʽΪ_______________________________________________������8.4 g B2���ɣ���ת�Ƶ�����Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������(BN)��һ����Ҫ�Ĺ����մɲ��ϡ�����Ȼ��ɰΪ��ʼ�����һϵ�з�Ӧ���Եõ�BF3��BN����ͼ��ʾ��
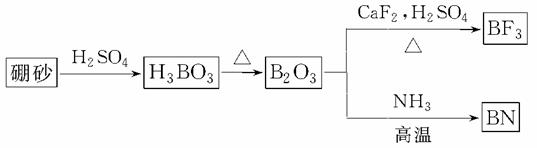
��ش��������⣺
(1)��B2O3�Ʊ�BF3��BN�Ļ�ѧ����ʽ������__________________________________��
___________________________________________________________________________��
(2)��̬B___________________________________________________________________��
B��N��ȣ��縺�Խϴ����________��BN��BԪ�صĻ��ϼ�Ϊ________��
(3)��BF3�����У�F—B—F�ļ�����________��Bԭ�ӵ��ӻ��������Ϊ________��BF3����NaF���ÿ�����NaBF4��BF �����幹��Ϊ____________��
�����幹��Ϊ____________��
(4)����ʯī�ṹ���Ƶ��������������У�����Bԭ����Nԭ��֮�仯ѧ��Ϊ____________�����������Ϊ___________________________________________________________________��
(5)�����������ڸ��¸�ѹ�£�����ת��Ϊ������������ṹ����ʯ���ƣ�Ӳ������ʯ�൱�������߳�Ϊ361.5 pm�������������к���________����ԭ�ӡ�________����ԭ�ӣ�������������ܶ���________ g·cm��3(ֻҪ������ʽ�����ؼ������ֵ�������ӵ�����ΪNA)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����
A��NaHCO3��H2 HCOONa��H2O��Ӧ�У����⡢������̾��������仯
HCOONa��H2O��Ӧ�У����⡢������̾��������仯
B����ͼ��ʾij���ȷ�Ӧ�ֱ����С�����������·�Ӧ�����е������仯
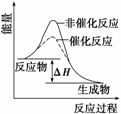
C����ѧ��Ӧ�����������ɣ�����ѭ�����غ㶨�ɺ������غ㶨��
D��2SO2(g)��O2(g)===2SO3(g)��4SO2(g)��2O2(g)===4SO3(g)�Ħ�H���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NA��ʾ�����ӵ�������ֵ������������ȷ����(����)
A������NA����ԭ�ӵĺ����ڱ�״���µ����ԼΪ11.2 L
B��25�棬1.01��105 Pa,64 g SO2�к��е�ԭ����Ϊ3NA
C���ڳ��³�ѹ�£�11.2 L Cl2���еķ�����Ϊ0.5 NA
D����״���£�11.2 L H2O���еķ�����Ϊ0.5 NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��0.01 mol�������ʷֱ����100 mL����ˮ�У��ָ������£�������Һ��������Ũ�ȵĴ�С˳����(��Һ����仯���Բ���)(����)
��Na2O2����Na2O����Na2CO3����NaCl
A����>��>��>�ܡ��������� B����>��>��>��
C���٣���>��>�� D���٣���>�ۣ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com