
����Ŀ���ҹ���ѧ��ʹ��˫���ܴ���(��������ͬ����)��ˮú���任��Ӧ��CO(g)��H2O(g)��CO2(g)��H2(g) ��H��0���ڵ����»�ýϸ߷�Ӧ���ʣ���Ӧ������ͼ��
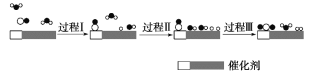
����˵����ȷ����
A.����֪CO��H2�ı�ȼ���ȣ��ɴ˿�������÷�Ӧ����H
B.���̢��̢��Ϊ���ȹ��̣������յ��������
C.�ڸ÷�Ӧ�����У�ʵ��������H2O���뷴Ӧ������������������
D.ʹ�ô���������ˮú���任��Ӧ����H������˷�Ӧ����
���𰸡�C
��������
A. ȼ������ָ1mol��ȼ����ȫȼ�������ȶ�������ʱ���ų�������������C��CO2(g)��H��H2O(l)�����д����CO��H2�ı�ȼ���ȵ��Ȼ�ѧ����ʽ�в�����H2O(g)�������������÷�Ӧ����H����A����
B. ��Ӧ�����ж��ѻ�ѧ�������������γɻ�ѧ���ų�����������I��II���л�ѧ���Ķ��ѣ����Զ�Ϊ���ȹ��̣����ǹ���I������CO���������̣����������仯����˹���I��II���յ���������ȣ���B����
C. ����ͼ֪���������������ֱ���һ��ˮ�����е����������ѣ�����������һ����������ͬʱ�γ�һ����������������һ��ˮ���ӣ������ڸ÷�Ӧ�����У�ʵ��������H2O���뷴Ӧ����������������������C��ȷ��
D. ʹ�ô������Խ��÷�Ӧ�Ļ�ܣ���߷�Ӧ���ʣ������ı��ʱ䣬��D����
��ѡC��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������������Ҫ���л��ϳ��м��壬��������ϩ��![]() ������
������![]() �ϳɻ����������·�ߣ�
�ϳɻ����������·�ߣ�
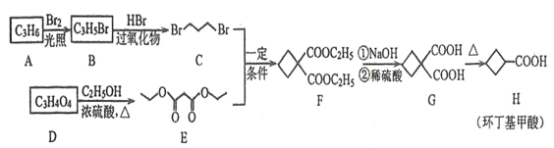
�ش��������⣺
(1)![]() �Ľṹ��ʽΪ______________��
�Ľṹ��ʽΪ______________��
(2)![]() �й����ŵ�����Ϊ______________��
�й����ŵ�����Ϊ______________��
(3)![]() �Ļ�ѧ����ʽΪ______________����Ӧ����Ϊ______________��
�Ļ�ѧ����ʽΪ______________����Ӧ����Ϊ______________��
(4)![]() �Ļ�ѧ����ʽΪ__________��
�Ļ�ѧ����ʽΪ__________��
(5)![]() �ķ���ʽΪ______��
�ķ���ʽΪ______��![]()
![]() ������̼��������Һ��Ӧ����
������̼��������Һ��Ӧ����![]() �����ʵ���Ϊ____
�����ʵ���Ϊ____![]() ��
��
(6)��������������![]() ������ͬ���칹��(�����������칹)����______�֣����к˴Ź���������3���Ľṹ��ʽΪ_________��
������ͬ���칹��(�����������칹)����______�֣����к˴Ź���������3���Ľṹ��ʽΪ_________��
����ʹ������Ȼ�̼��Һ��ɫ
������![]() ��Һ��Ӧ
��Һ��Ӧ
������������Һ����������Ӧ
�ܲ�����״�ṹ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1���ڢ�CO2����NaCl����Na����Si����CS2�����ʯ���ߣ�NH4��2SO4�����Ҵ��У��ɼ��Լ��γɵķǼ��Է�����_______������ţ�����ͬ�������н������ӵ�������__�����Ӽ���γ������������________���������Ӿ������__������ԭ�Ӿ������__���١����������ʵ��۵��ɸߵ��͵�˳����__��
��2��A��B��C��DΪ���־��壬�������£�
A����̬ʱ�ܵ��磬����������
B��������CS2��������ˮ
C����̬ʱ�����磬Һ̬ʱ�ܵ��磬������ˮ
D����̬��Һ̬ʱ�������磬�۵�Ϊ3500 ��
���ƶ����ǵľ������ͣ�A��__��B��__��C��__��D��__��
��3����ͼ��A��D����ѧ��ѧ�̿����ϳ����ļ��־���ṹģ�ͣ�����д��Ӧ���ʵ����ƣ�A��__��B��__��C��__D��____��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��s�ܼ���p�ܼ��ĵ���������ͼ���Իش����⡣


(1)s����������ͼ��________�Σ�ÿ��s�ܼ���________��ԭ�ӹ����p����������ͼ��________״��ÿ��p�ܼ���________��ԭ�ӹ������������ϵΪ____________(���ͬ������ͬ��)��
(2)Ԫ��X��ԭ�������ĵ����Ų�ʽΪnsnpn��1��ԭ����������ߵ���________���ӣ�Ԫ��X��������____�������⻯��ĵ���ʽ��________��
(3)��Ԫ��Y��ԭ�������ĵ����Ų�ʽΪnsn��1npn��1����ôY��Ԫ�ط���ӦΪ________��ԭ�ӵĵ����Ų�ͼΪ______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������̼���ɼ��������ͼ��ʾ��NAΪ�����ӵ�������ֵ������˵����ȷ����
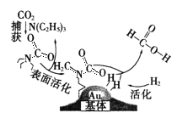
A.10.1gN(C2H5)3������һ���������γ��Σ�����[N(C2H5)3H]+����ĿΪ0.1NA
B.30g��������У����Ӷ���ĿΪ5 NA
C.�ڲ�������У�����22.4L CO2����״�������뷴Ӧ�����ڷ�Ӧ������CO2����ֻ����NA�Ե��Ӷ�
D.�÷�Ӧ��������ԭ��Ӧ��ÿ����1mol���ᣬת�Ƶĵ�����Ϊ2NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1������֮��Ϊ16��7��6����������SO2��CO��NO�����Ӹ���֮��Ϊ__����ԭ�Ӹ���֮��Ϊ___��
��2��ij����������Ļ�ѧʽΪRO2���ڱ�״���£�0.92g������������Ϊ448mL������������Ħ������Ϊ___��R�����ԭ������Ϊ__��
��3�������£���27.5gˮ���ܽ�12.5gCuSO4��5H2O��ǡ�ôﵽ���ͣ�����Һ���ܶ�Ϊ1.21g/cm3�������Һ��CuSO4�����ʵ���Ũ��ԼΪ__������ȡ��20.0mL����Һ�����Ũ��Ϊ1.00mol/L��ϡ��Һ����ϡ�ͺ���Һ�������__mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ����ˮ�ʼ��վ����480 mL 0��5 mol/L NaOH��Һ�Ա�ʹ�á�
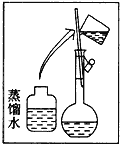
(1)��ͬѧӦѡ��____ mL������ƿ��
(2)�����������ͼ��ʾ����ͼ�в���Ӧ����ͼ�е�____����ѡ����ĸ��֮�䡣
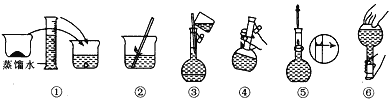
A������� B������� C�������
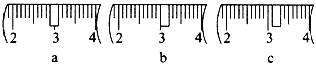
(3)��ͬѧӦ��ȡNaOH����_____g��������Ϊ23.1 g���ձ�����������ƽ�ϳ�ȡ����NaOH����ʱ�����ڸ�����ѡȡ����������С____������ĸ����������ͼ��ѡ������ȷ��ʾ����λ�õ�ѡ��____������ĸ����

(4)���в�����������Һ��Ũ�ȴ�С�к�Ӱ�죿
��ת������Һ��δϴ�Ӳ��������ձ���Ũ�Ȼ�________���ƫ����ƫС������Ӱ�족����ͬ��
������ƿ��ԭ������������ˮ��Ũ�Ȼ�_______����Һδ��ȴ��ת��������ƿ____________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25��ʱ��ȡ0.l molL-1 HA��Һ��0.1 molL-1 NaOH��Һ�������ϣ���Ϻ���Һ����ı仯���ƣ�����û����Һ��pH��9���Իش��������⣺
��1�������Һ��pH��9��ԭ��_____________________�������ӷ���ʽ��ʾ����
��2�������Һ����ˮ�������c(OH��) =______molL-1 ��0.1 molL-1NaOH ��Һ����ˮ�������c(OH��) =______molL-1 ��
��3��0.l molL-1 HA��Һ��0.05 molL-1 NaOH��Һ�������Ϻ�pH��8����
�� ���û��Һ��c(HA)��c(A��)��c(Na+)��c(OH��)��c(H+) Ũ�ȴӴ�С��˳��Ϊ��_________________��
�� c(HA)+ c(A��)��_______ molL-1��c(HA)��c(A��)��_______molL-1��
��4��25��ʱ����֪NH4A��ҺΪ���ԣ���HA��Һ�ӵ�Na2CO3��Һ��������ų������ƶ�(NH4)2CO3��Һ�� pH________7������������������������������
��5����ͬ�¶�����ͬ���ʵ���Ũ�ȵ�������������Һ��pH�ɴ�С��˳��____������ĸ����
A��NH4HCO3 B��NH4HSO4 C��NH4A D��NH4Cl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵�����ʾ��������ȷ����
A. ������ȼ����Ϊ285.8 kJ��mol��1��������ȼ�յ��Ȼ�ѧ����ʽΪ2H2(g)��O2(g)=2H2O(l) ��H����285.8 kJ��mol��1
B. ������������������۷ֱ���ȫȼ�գ����߷ų���������
C. ij�ܱ�����ʢ��0.1 mol N2��0.3 mol H2����һ�������³�ַ�Ӧ,ת�Ƶ��ӵ���ĿС��0.6��6.02��1023
D. ��֪�к���Ϊ57.3 kJ��mol��1��������0.5 mol H2SO4��Ũ������Һ�뺬1 mol NaOH����Һ��ϣ��ų�������ҪС��57.3 kJ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com