
����Ŀ��������ͼװ��̽��ͭ���������Լ��ķ�Ӧ�����Թ��м���һ����ͭ�ۣ������Լ�����������������ʵ���������±���ʾ�������ƶ�����ȷ����( )
���ϣ�Cu2++4NH3![]() [Cu(NH3)4]2+, [Cu(NH3)4]2+�����ɫ��
[Cu(NH3)4]2+, [Cu(NH3)4]2+�����ɫ��
װ�� | ��� | �Լ� | ���� |
| �� | 10 mLŨNH3��H2O��3 mLH2O | ��Һ��Ϊ���ɫ�����ˮ������ |
�� | 10 mLŨNH3��H2O��3 mL H2O2 | ��Һ��Ϊ���ɫ���ұȢ��� | |
�� | 10 mL 20% HNO3��Һ��3 mL H2O | ��Һ���ٱ�����������ɫ���壬���ˮ���½� | |
�� | 10 mL 20% HNO3��Һ��3 mL H2O2 | ��Һ�����Ȣ�����������ɫ���壬���ˮ���½� |
A.����ʵ������˵��O2�����˷�Ӧ
B.������Һ��ɫ�Ȣ������������H2O2����Cu����Cu2+
C.�������ˮ���½������Ƿ�Ӧ����NO���嵼�µ�
D.�ܱȢ۱�����������H2O2�ֽ������������
���𰸡�D
��������
����Ϊ̽��ʵ�飬����ʵ����̼�ʵ�����������������ƶϣ���ͭ������Ҫ��ǿ����������ʵ���Ũ��ˮ��������ͭ����˿��Ʋ����������������뷴Ӧ��ʵ����й���������ǿ���ԣ�����һ�������½�ͭ���������������ǿ�����ԣ��������ܽ�ͭ���������������������������������ͬʱʹ��Ҫ�����֮���Ӱ�졣
A���Һ��Ϊ���ɫ��˵��ͭ�����������ˮ��������˵��װ����������١��ɴ��Ʋ⣬װ���ڵ�������Ũ��ˮ�Ļ����½�ͭ������Aѡ����ȷ��
B�������Һ��ɫ�Ȣ��е��˵�����ɵĺ�ͭ������Ũ�ȸ��ߣ��Աȿ�֪�����ڹ�������Ĵ��ڣ�������ͭ���ܽ�����Bѡ����ȷ��
C��������������ǿ�����Կ�֪��ͭ���������������ᱻ��ԭ��NO��Cѡ����ȷ��
D�����ʵ�������֪����������Ĵ��ڶ�������ͭ��������Ӧ���˸������á�����Ļ�ԭ����ΪNO�������������ֽ����O2�������NO��Ӧ���ɺ���ɫ��NO2����ʵ�����������Dѡ�����
��ѡD��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������(SiHCl3)���Ʊ����顢�ྦྷ�����Ҫԭ�ϡ��ش��������⣺
(1)SiHCl3�ڴ��������·�����Ӧ��
2SiHCl3(g)=SiH2Cl2(g)+ SiCl4(g) ��H1=48 kJ��mol-1
3SiH2Cl2(g)=SiH4(g)+2SiHCl3 (g) ��H2=30 kJ��mol-1
��Ӧ4SiHCl3(g)=SiH4(g)+3SiCl4(g)�Ħ�H=__________kJ��mol-1��
(2)���ڷ�Ӧ2SiHCl3(g)=SiH2Cl2(g)+SiCl4(g)�����ô�������������ӽ�����֬��������323K��343KʱSiHCl3��ת������ʱ��仯�Ľ����ͼ��ʾ��
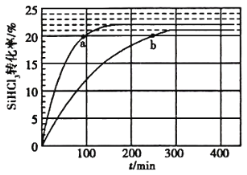
�� 343Kʱ��Ӧ��ƽ��ת���ʦ�=__________%��ƽ�ⳣ������ʽ__________��
����343K�£�Ҫ���SiHCl3ת���ʣ��ɲ�ȡ�Ĵ�ʩ��__________��Ҫ���̷�Ӧ�ﵽƽ���ʱ�䣬�ɲ�ȡ�Ĵ�ʩ��__________��__________��
�۱Ƚ�a��b����Ӧ���ʴ�С��va__________vb(����ڡ���С�ڡ����ڡ�)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��п������п�����̵��ң���ZnO 35%���ϣ�������������������(MnO)������ͭ�� ����������Ͳ�����������ʣ���ҵ�ϳ������������ ZnSO4��7H2O����֪ ZnSO4��7H2O ����������ˮ�������ھƾ���ij��ȤС��ʵ����ģ����� ZnSO4��7H2O ���壬�������£�

��ش�
(1)�������� II �еIJ�����ԭ���� �ش��������⣺
�ٽ�ϱ� 1�� 2������ѡ��� pH ���¶ȷֱ���______________________�� ���У� ���Բ��ü���_________________________������ pH ��
��1 pH��ZnSO4.7H2O �����������ȵ�Ӱ��
pH | ZnSO4.7H2O ��������g�� | ��Ʒ��Fe�ĺ���% | ��Ʒ�������ؽ�������% |
1 | 114.32 | 0.750 | 0.059 |
2 | 114.4 | 0.086 | 0.056 |
3 | 113.68 | 0.034 | 0.054 |
4 | 113.60 | 0.010 | 0.050 |
5 | 112.43 | 0.010 | 0.050 |
��2 �¶ȶ�ZnSO4.7H2O �����������ȵ�Ӱ��
�¶� (��) | ZnSO4.7H2O ��������g�� | ��Ʒ��F�ĺ���% | ��Ʒ�������ؽ�������% |
20 | 111.45 | 0.011 | 0.052 |
40 | 112.89 | 0.010 | 0.051 |
60 | 113.30 | 0.010 | 0.050 |
80 | 113.80 | 0.010 | 0.050 |
90 | 114.40 | 0.091 | 0.048 |
������ KMnO4 ��Һ����Һ�е� Fe2+�������������ֳ�����ͬʱ�������ĸ�����������Ե��������Զ��ֽ�����MnO2 ��������д���ڸû����£�KMnO4 ��Һ���� Fe2+�����ӷ�Ӧ����ʽ_________________________________________�� ����ϡ�����������������˲����п��ܺ��� Zn(NO3)2 �⣬�����ܵ�ȱ���ǣ�_________________________��
(2)��������ʵ������У��ش��������⣺
������ B ����Ҫ�ɷ�Ϊ___________________________��
����μ�����ҺB���Ƿ�����Ԫ��_____________________________________��
��д����������C�����ӷ���ʽ__________________________________________��
(3)Ϊ�ⶨ ZnSO4��7H2O ����Ĵ��ȣ����� K4Fe(CN)6 ��Һ���еζ�����Ҫԭ�����£�2K4Fe(CN)6+ 3ZnSO4= K2Zn3[Fe(CN)6]2��+ 3K2SO4
ȷ��ȡ 5.000g ZnSO4��7H2O ���壬������ˮ�ܽⲢ������ 250mL��ȷ��ȡ����Һ 25.00mL����ƿ�У��� 0.0500mol/L K4Fe(CN)6 ��Һ���еζ��������������±���
ʵ����� | �ζ�ǰ����/mL | �ζ������/mL |
1 | 0.10 | 19.92 |
2 | 1.34 | 21.12 |
3 | 0.00 | 20.10 |
�� ZnSO4��7H2O ����Ĵ�����_______________(������������ʾ��������С�������λ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ�˷�ֹǹ֧���⣬����ǹ֧�ĸ����������NaNO2��NaOH�Ļ����Һ�н��л�ѧ����ʹ���������������Fe3O4�����ܵı�����������������������̿������л�ѧ����ʽ��ʾ��
��3Fe��NaNO2��5NaOH===3Na2FeO2��H2O��NH3��
��![]() Na2FeO2��
Na2FeO2��![]() NaNO2��
NaNO2��![]() H2O�D��
H2O�D��![]() Na2Fe2O4��
Na2Fe2O4��![]() NH3����
NH3����![]() NaOH
NaOH
��Na2FeO2��Na2Fe2O4��2H2O![]() Fe3O4��4NaOH
Fe3O4��4NaOH
��ش�����������
��1����ƽ��ѧ����ʽ����������ǰ�Ļ�ѧ����������Ϊ_____________��
��2��������Ӧ����������Ϊ______����������������______������2 mol Na2FeO2���ɣ���Ӧ������________mol���ӷ���ת�ơ�
��3���������������Ĺ��̣�����˵������ȷ����________(����ĸ)��
A���ù��̲��������Ⱦ
B����Ӧ�����ɵ��������������п���ʴ����
C����Ӧ�٢ڢ۾���������ԭ��Ӧ
D����Ӧ�٢��е���������ΪNaNO2
��4���������Һ��NaOHŨ�ȹ������������ĺ�Ȼ��С����ԭ����________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������仯������;�dz��㷺���ش��������⣺
��1�����ķ���ϩ��һ�����壬������һ����ƽ�����������ϸ�����λ����Ķ��ؾ��壬��ͨ��___�������־�������ͷǾ��塣
��2��[H2F]+[SbF6]-�������ᣩ��һ�ֳ�ǿ�ᣬ����[H2F]+�������ӵĿռ乹��Ϊ___��
��3��NH4F������泥������ڲ�����ʴ�̡������������������������Һ�д���__������ĸ����
a.���Ӽ� b.���� c.���� d.��� e.��λ��
��4��SF6���㷺������ѹ�����豸�ľ�Ե���ʡ�SF6��һ�ֹ��ۻ������ͨ��������Born-Haberѭ��������������ͼ������ؼ��ܡ���F-F���ļ���Ϊ____kJmol-1��
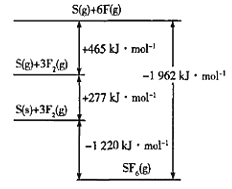
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������ͿƼ��������ش����á�Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч����ij��ѧ�о�С���ͬѧ�ֱ��������ͼ�ס�����ʾ��ʵ�顣��ش�������⣺

(1)������ͼ��ͨ���۲�_____________�����ԱȽϵó����ۡ�ͬѧX�۲������֧�Թܲ������ݵĿ������ɴ˵ó�Fe3����Cu2����H2O2�ֽ�Ĵ�Ч���������__________(����������������������)��������___________
(2)������ͼ����ʾ��ʵ��ʱ��������40 mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԡ�ʵ������Ҫ������������_____________________��
(3)����0.10 mol MnO2��ĩ��50 mL H2O2��Һ�У��ڱ�״���·ų�����������ʱ��Ĺ�ϵ��ͼ��ʾ��
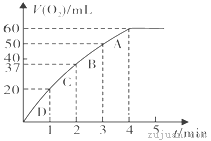
��ʵ��ʱ�ų�������������__________mL��
�ڷų�1/3��������ʱ��Ϊ___________min��
�ۼ���H2O2�ij�ʼ���ʵ���Ũ��_______________�� (�뱣����λ��Ч����)
��A��B��C��D���㷴Ӧ���ʿ�����˳��Ϊ_____��____��____��____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ԫ�����ڱ�����ʽ���ֶ�������ͼ������Ԫ�����ڱ���һ����(1��36��Ԫ��)���Ա���ѧ����Ԫ�����ڱ���˼������Ԫ�����ڱ��������ɣ��Ƴ�ͼ�б�ǵ�11��Ԫ�أ��ش��������⣺
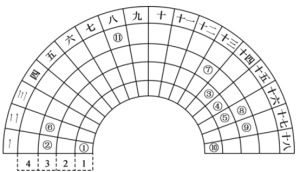
(1)�ݵļ������ӵĽṹʾ��ͼΪ_______����ԭ������Ϊ___________��
(2)�ܵļ��⻯��ĵ���ʽΪ__________________��
(3)��11��Ԫ���У����ʵĻ�ѧ��������õ���__________(�ѧʽ)��
(4)�ࡢ������Ԫ���γɵ�����������ˮ�����У����Խ�ǿ����_______(�ѧʽ)��
(5)����ʱ���۵ĵ����ܺ͢������������ˮ�����Ũ��Һ������Ӧ����ѧ����ʽΪ___________��
(6)����Ԫ�����ڱ��е�λ����_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����б�ʾ��Ӧ��ѧ��Ӧ�����ӷ���ʽ��ȷ���ǣ� ��
A.MnO2��Ũ���ᷴӦ��Cl2��MnO2+4HCl![]() Mn2��+2Cl��+Cl2��+2H2O
Mn2��+2Cl��+Cl2��+2H2O
B.��������ˮ����Al(OH)3���壺Al3��+3H2O =Al(OH)3��+3H��
C.Na2O2����ˮ����O2��Na2O2+H2O =2Na��+2OH��+O2��
D.Ca(HCO3)2��Һ������NaOH��Һ��Ӧ��HCO3��+Ca2��+OH��= CaCO3��+H2O
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com