
ЁОЬтФПЁПЯТБэЪЧдЊЫижмЦкБэЕФвЛВПЗжЃЌБэжаЕФЪ§зжБэЪОвЛжжЖЬжмЦкдЊЫиЃЌЛиД№ЯТСаЮЪЬтЃК

ЃЈ1ЃЉЛГіЂлЕФдзгНсЙЙЪОвтЭМЃК____________________ЁЃ
ЃЈ2ЃЉЂлЁЂЂмЁЂЂпЁЂЂрЫФжждЊЫиЫљаЮГЩЕФЦјЬЌЧтЛЏЮяжазюЮШЖЈЕФЪЧ__________ЃЈЬюЛЏбЇЪНЃЉЁЃ
ЃЈ3ЃЉЂйдкдЊЫижмЦкБэжаЕФЮЛжУЪЧ____________________________ЁЃ
ЃЈ4ЃЉЂрЁЂЂсдЊЫиЕФзюИпМлбѕЛЏЮяЖдгІЫЎЛЏЮяЕФЫсадЃК____>____ЃЈгУЛЏбЇЪНБэЪОЃЉЁЃ_________
ЃЈ5ЃЉгЩЂлЁЂЂнКЭЂтзщГЩЕФЛЏКЯЮяЫљКЌЛЏбЇМќРраЭЮЊ__________ЃЌЕчзгЪНЮЊ____________ЁЃ
ЃЈ6ЃЉЂмЁЂЂнЁЂЂоЕФдзгАыОЖгЩДѓЕНаЁЕФЫГађЪЧЃК____>____>____ЃЈгУдЊЫиЗћКХБэЪОЃЉЁЃ__________
ЃЈ7ЃЉЂоЕЅжЪгыЧтбѕЛЏФЦШмвКЗДгІЕФЛЏбЇЗНГЬЪНЪЧ__________________________________ЁЃ
ЃЈ8ЃЉвбжЊЛЏКЯЮяAЁЂBЁЂCЁЂDЁЂEЁЂFЁЂGКЭЕЅжЪМзЁЂввЫљКЌдЊЫиОљЮЊЩЯБэжадЊЫизщГЩЁЃ
ЂйAЕФХЈШмвКгыМзФмЗЂЩњШчЯТЭМЫљЪОЕФЗДгІЁЃ
ЂкМзЪЧГЃМћЕФКкЩЋЙЬЬхЕЅжЪЃЌПЩЮЊЩњВњЩњЛюЬсЙЉШШФмЁЃ
ЂлввЪЧГЃМћЕФЮоЩЋЦјЬхЕЅжЪЁЃ
ЂмBЪЧЮоЩЋгаДЬМЄадЦјЮЖЕФЦјЬхЃЌЪЧжївЊЕФДѓЦјЮлШОЮяжЎвЛЁЃ
ЂнГЃЮТЯТЃЌCЪЧвЛжжЮоЩЋвКЬхЁЃ

ЛиД№ЮЪЬтЃК
ЂйаДГіЯТСаЮяжЪЕФЛЏбЇЪНЃКA_____ЃЌE_____ЃЌG_____ЁЃ
ЂкаДГіЯТСаЗДгІЕФЛЏбЇЗНГЬЪНЃК
CЃЋEЁњFЃЋвв________________________________ЁЃ
BЃЋCЃЋввЁњA________________________________ЁЃ
ЁОД№АИЁП![]() HF ЕкЖўжмЦкIVAзх HClO4 H2SO4 РызгМќЁЂЙВМлМќ
HF ЕкЖўжмЦкIVAзх HClO4 H2SO4 РызгМќЁЂЙВМлМќ ![]() Na Al F 2AlЃЋ2OHЃЃЋ2H2OЃН2AlO2ЃЃЋ3H2Ёќ H2SO4 Na2O2 Na2CO3 2Na2O2ЃЋ2H2OЃН4NaOHЃЋO2Ёќ 2SO2ЃЋO2ЃЋ2H2OЃН2H2SO4
Na Al F 2AlЃЋ2OHЃЃЋ2H2OЃН2AlO2ЃЃЋ3H2Ёќ H2SO4 Na2O2 Na2CO3 2Na2O2ЃЋ2H2OЃН4NaOHЃЋO2Ёќ 2SO2ЃЋO2ЃЋ2H2OЃН2H2SO4
ЁОНтЮіЁП
ИљОндЊЫижмЦкБэжЊЃЌЂйЂкЂлЂмЂнЂоЂпЂрЂсЂтЫљДњБэЕФдЊЫиЗжБ№ЮЊCЁЂNЁЂOЁЂFЁЂNaЁЂAlЁЂPЁЂSЁЂClЁЂHЁЃ
ЃЈ1ЃЉЂлЪЧOдЊЫиЃЌOдзгжЪзгЪ§=КЫЭтЕчзгЪ§=8ЃЌKВу2ИіЕчзгЁЂLВу6ИіЕчзгЃЌдзгНсЙЙЪОвтЭМЃК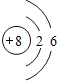 ЃЌЙЪД№АИЮЊЃК
ЃЌЙЪД№АИЮЊЃК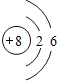 ЃЛ
ЃЛ
ЃЈ2ЃЉЂлЁЂЂмЁЂЂпЁЂЂрЫФжждЊЫиЗжБ№ЪЧOЁЂFЁЂPЁЂSЃЌЗЧН№ЪєаддНЧПЦфЦјЬЌЧтЛЏЮядНЮШЖЈЃЌЦфжаЗЧН№ЪєадзюЧПЕФдЊЫиЪЧFЃЌвђДЫHFзюЮШЖЈЃЌЙЪД№АИЮЊЃКHFЃЛ
ЃЈ3ЃЉЂйЪЧCдЊЫиЃЌдкдЊЫижмЦкБэжаЕФЮЛжУЪЧЕкЖўжмЦкIVAзхЃЌЙЪД№АИЮЊЃКЕкЖўжмЦкIVAзхЃЛ
ЃЈ4ЃЉЂрЁЂЂсдЊЫиЗжБ№ЪЧSЁЂClЃЌЗЧН№ЪєадClЃОSЃЌЗЧН№ЪєаддНЧПЃЌзюИпМлбѕЛЏЮяЖдгІЫЎЛЏЮяЕФЫсаддНЧПЃЌвђДЫЫсадЃКHClO4ЃОH2SO4ЃЌЙЪД№АИЮЊЃКHClO4ЃЛH2SO4ЃЛ
ЃЈ5ЃЉЂлЁЂЂнКЭЂтдЊЫиЗжБ№ЪЧOЁЂNaЁЂHЃЌгЩЂлЁЂЂнКЭЂтзщГЩЕФЛЏКЯЮяЪЧNaOHЃЌNaOHЪЧРызгЛЏКЯЮяЃЌЫљКЌЛЏбЇМќРраЭЮЊРызгМќКЭЙВМлМќЃЌЕчзгЪНЮЊЃК![]() ЃЌЙЪД№АИЮЊЃКРызгМќЁЂЙВМлМќЃЛ
ЃЌЙЪД№АИЮЊЃКРызгМќЁЂЙВМлМќЃЛ![]() ЃЛ
ЃЛ
ЃЈ6ЃЉЂмЁЂЂнЁЂЂоЗжБ№ЪЧFЁЂNaЁЂAlЃЌИљОнЭЌжмЦкздзѓЕНгвдзгАыОЖж№НЅМѕаЁЃЌдзгАыОЖЃКNaЃОAlЃОClЃЌЭЌжїзхздЩЯЖјЯТдзгАыОЖж№НЅдіДѓЃЌдзгАыОЖЃКClЃОFЃЌвђДЫдзгАыОЖЃКNaЃОAlЃОFЃЌЙЪД№АИЮЊЃКNaЃЛAlЃЛFЃЛ
ЃЈ7ЃЉЂоЪЧAlдЊЫиЃЌAlгыЧтбѕЛЏФЦШмвКЗДгІЕФРызгЗНГЬЪНЮЊ2AlЃЋ2OHЃЃЋ2H2OЃН2AlO2ЃЃЋ3H2ЁќЃЛ
ЃЈ8ЃЉЂйгЩЗДгІЙиЯЕЭМПЩжЊЃКМзЪЧГЃМћЕФКкЩЋЙЬЬхЕЅжЪЃЌПЩЮЊЩњВњЩњЛюЬсЙЉШШФмЃЌПЩжЊМзЪЧCЃЌBЪЧЮоЩЋгаДЬМЄадЦјЮЖЕФЦјЬхЃЌЪЧжївЊЕФДѓЦјЮлШОЮяжЎвЛЃЌЮЊSO2ЦјЬхЃЌвђДЫAЮЊХЈСђЫсШмвКЃЌГЃЮТЯТЃЌCЪЧвЛжжЮоЩЋвКЬхЪЧЫЎЃЌDЮЊCO2ЃЌЖўбѕЛЏСђКЭбѕЦјКЭЫЎЗДгІЩњГЩСђЫсЃЌЙЪввЮЊO2ЃЌЫЎЁЂЖўбѕЛЏЬМОљгыEЗДгІЩњГЩбѕЦјЃЌЫЕУїEЮЊNa2O2ЃЌдђFЮЊNaOHЃЌGЮЊNa2CO3ЃЌЙЪД№АИЮЊЃКH2SO4ЃЛNa2O2ЃЛNa2CO3ЃЛ
ЂкЫЎКЭЙ§бѕЛЏФЦЗДгІЩњГЩЧтбѕЛЏФЦКЭбѕЦјЃЌЛЏбЇЗНГЬЪНЮЊЃК2Na2O2+2H2O=4NaOH+O2ЁќЃЌЖўбѕЛЏСђКЭбѕЦјКЭЫЎЗДгІЩњГЩСђЫсЃЌЛЏбЇЗНГЬЪНЮЊЃК2SO2+O2+2H2O=2H2SO4ЃЌЙЪД№АИЮЊЃК2Na2O2+2H2O=
4NaOH+O2ЁќЃЛ2SO2+O2+2H2O=2H2SO4ЁЃ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПгаЛњввХМвіДцдкгкЦЁОЦжаЃЌЪЧОЦРрЕїЯужавЛИіМЋЦфживЊЕФЦЗжжЃЌФГбаОПадбЇЯАаЁзщЮЊШЗЖЈввХМвіЕФНсЙЙЃЌНјааШчЯТЬНОПЁЃ
ВНжшвЛЃКНЋввХМвіеєЦјЭЈЙ§ШШЕФбѕЛЏЭ(ДпЛЏМС)бѕЛЏГЩЖўбѕЛЏЬМКЭЫЎЃЌдйгУзАгаЮоЫЎТШЛЏИЦКЭЙЬЬхЧтбѕЛЏФЦЕФЮќЪеЙмЭъШЋЮќЪеЃЌШчЭМ1ЁЃ2.64 gввХМвіЕФеєЦјбѕЛЏВњЩњ5.28 gЖўбѕЛЏЬМКЭ2.16 gЫЎЁЃ
ВНжшЖўЃКЩ§ЮТЪЙввХМвіЦћЛЏЃЌВтЦфУмЖШЪЧЯрЭЌЬѕМўЯТH2ЕФ44БЖ
ВНжшШ§ЃКгУКЫДХЙВеёвЧВтГіввХМвіЕФКЫДХЙВеёЧтЦзШчЭМ2ЃЌЭМжа4ИіЗхЕФУцЛ§БШЮЊ1ЁУ3ЁУ1ЁУ3ЁЃ
ВНжшЫФЃКРћгУКьЭтЙтЦзвЧВтЕУввХМвіЗжзгЕФКьЭтЙтЦзШчЭМ3ЁЃ



ЃЈ1ЃЉЭМ1зАжУжаСНжЇUаЭЙмВЛФмЛЅЛЛЕФРэгЩЪЧ__________________________ЃЎ
ЃЈ2ЃЉввХМвіЕФФІЖћжЪСПЮЊ____________ЁЃ
ЃЈ3ЃЉввХМвіЕФЗжзгЪНЮЊ____________________ЁЃ
ЃЈ4ЃЉввХМвіЕФНсЙЙМђЪНЮЊ ________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЙигк![]() (Тн[2ЃЌ2]ЮьЭщ)ЕФЫЕЗЈе§ШЗЕФЪЧ
(Тн[2ЃЌ2]ЮьЭщ)ЕФЫЕЗЈе§ШЗЕФЪЧ
A. вЛТШДњЮяЕФНсЙЙжЛгавЛжж
B. гыЮьЯЉЛЅЮЊЭЌЗжвьЙЙЬх
C. ЫљгаЬМдзгОљДІЭЌвЛЦНУц
D. ФмЪЙЫсадИпУЬЫсМиШмвКЭЪЩЋ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЖўМзУб(CH3OCH3)БЛГЦЮЊ21ЪРМЭЕФаТаЭШМСЯЃЌвдCOКЭH2ЮЊдСЯЩњВњЖўМзУбжївЊЗЂЩњвдЯТШ§ИіЗДгІЃК
ЃЈ1ЃЉИУЙЄвеЕФзмЗДгІЮЊ3CO(g)ЃЋ3H2(g)![]() CH3OCH3(g)ЃЋCO2(g) ІЄHЃН_________ЃЌЛЏбЇЦНКтГЃЪ§KЃН______________(гУКЌK1ЁЂK2ЁЂK3ЕФДњЪ§ЪНБэЪО)ЁЃ
CH3OCH3(g)ЃЋCO2(g) ІЄHЃН_________ЃЌЛЏбЇЦНКтГЃЪ§KЃН______________(гУКЌK1ЁЂK2ЁЂK3ЕФДњЪ§ЪНБэЪО)ЁЃ
ЛЏбЇЗДгІЗНГЬЪН | ЛЏбЇЦНКтГЃЪ§ | |
ЂйCO(g)ЃЋ2H2(g) | ІЄH1=-99 kJmol-1 | K1 |
Ђк2CH3OH(g) | ІЄH2ЃНЃ24 kJmol-1 | K2 |
ЂлCO(g)ЃЋH2O(g) | ІЄH3ЃНЃ41 kJmol-1 | K3 |
ЃЈ2ЃЉФГЮТЖШЯТЃЌНЋ8.0molH2КЭ4.0molCOГфШыШнЛ§ЮЊ2LЕФУмБеШнЦїжаЃЌЗЂЩњЗДгІЃК4H2(g)+2CO(g) ![]() CH3OCH3(g)+H2O(g)ЃЌ10 ЗжжгКѓЗДгІДяЦНКтЃЌВтЕУЖўМзУбЕФЬхЛ§ЗжЪ§ЮЊ25%ЃЌдђгУH2БэЪОЕФЗДгІЫйТЪЮЊ_________ЃЌCOЕФзЊЛЏТЪЮЊ________ЁЃ
CH3OCH3(g)+H2O(g)ЃЌ10 ЗжжгКѓЗДгІДяЦНКтЃЌВтЕУЖўМзУбЕФЬхЛ§ЗжЪ§ЮЊ25%ЃЌдђгУH2БэЪОЕФЗДгІЫйТЪЮЊ_________ЃЌCOЕФзЊЛЏТЪЮЊ________ЁЃ
ЃЈ3ЃЉЯТСаДыЪЉжаЃЌФмЬсИпCH3OCH3ВњТЪЕФга________ЁЃ
AЃЎЗжРыГіЖўМзУб BЃЎНЕЕЭЮТЖШ CЃЎИФгУИпаЇДпЛЏМСDЃЎдіДѓбЙЧП
ЃЈ4ЃЉИУЙЄвежаЗДгІЂлЕФЗЂЩњЬсИпСЫCH3OCH3ЕФВњТЪЃЌдвђЪЧ________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯжгаЫФзщЗжЩЂЯЕЃКЂйЦћгЭКЭЫЎаЮГЩЕФЛьКЯвКЂкКЌгаФрЩГЕФЪГбЮЫЎЂлШмгаЕт(I2)ЕФТШЛЏМиШмвКЂмввЖўДМКЭБћШ§ДМЛьКЯШмвК(ввЖўДМКЭБћШ§ДМЕФВПЗжЮяРэаджЪМћБэ)ЁЃ
ЮяжЪ | ШлЕу/Ёц | ЗаЕу/Ёц | УмЖШ/gcm-3 | ШмНтад |
ввЖўДМ | 11.5 | 198 | 1.11 | взШмгкЫЎКЭввДМ |
БћШ§ДМ | 17.9 | 290 | 1.26 | ФмИњЫЎЁЂОЦОЋвдШЮвтБШЛЅШм |
ЧыгУШчЭМЫљЪОЕФвЧЦїЗжРывдЩЯИїЛьКЯвКЃЌвЧЦїКЭЗНЗЈВЛФмЖдгІЕФЪЧЃЈ ЃЉ

A. ЂйЁЊcЁЊЗжвКB. ЂкЁЊaЁЊнЭШЁ
C. ЂлЁЊcЁЊнЭШЁD. ЂмЁЊaЁЊеєСѓ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТБэЪЧдЊЫижмЦкБэЕФвЛВПЗжЃЌЧыЛиД№гаЙиЮЪЬт:

ЃЈ1ЃЉБэжаЛЏбЇаджЪзюВЛЛюЦУЕФдЊЫиЃЌЦфдзгНсЙЙЪОвтЭМЮЊ______ЁЃ
ЃЈ2ЃЉдЊЫиЂпгыЂрЕФдзгАыОЖДѓаЁЙиЯЕЪЧЃКЂп______Ђр(ЬюЁАЃОЁБЛђЁАЃМЁБЃЉЁЃ
ЃЈ3ЃЉЂмЂнСНдЊЫиЯрБШНЯЃЌН№ЪєадНЯЧПЕФЪЧ______ (ЬюдЊЫиУћГЦЃЉЁЃ
ЃЈ4ЃЉдЊЫиЂйЕФзюИпМлбѕЛЏЮяЕФЫЎЛЏЮяЕФЛЏбЇЪНЮЊ________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЫЕЗЈжае§ШЗЕФЪЧЃЈ ЃЉ
A. Аб100mL3mo/LH2SO4ШмвКгы100mL1mo/LBaCl2ШмвКЛьКЯЃЌЫљЕУШмвКc(SO42-)БфЮЊ1mol/L
B. Аб200mL3mol/LBaCl2ШмвКгы100mL3mol/LKCl ШмвКЛьКЯКѓЃЌЫљЕУШмвКc(Cl-)ШдЮЊ3mol/L
C. Аб100mL20%ЕФNaOHШмвКгы100mLH2OЛьКЯКѓЃЌЫљЕУШмвКжаNaOHЕФжЪСПЗжЪ§ЮЊ10%
D. Аб100g20%ЕФNaClШмвКгы100mLH2OЛьКЯКѓЃЌЫљЕУШмвКжаNaClЕФжЪСПЗжЪ§ЮЊ10%
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПИпЗжзгЛЏКЯЮяGЪЧПЩБЛЯИОњзїЮЊЬМдДКЭФмдДРћгУЕФОлКЯЮяЃЌЪєгквЛжжЩњЮяПЩНЕНтИпЗжзгВФСЯЃЌдкЪГЦЗЁЂвЉЦЗАќзАЗНУцОпгаЖРЬигХЪЦЁЃвбжЊA~GОљЮЊгаЛњЛЏКЯЮяЃЌвдЯТЮЊИпЗжзгЛЏКЯЮяGЕФвЛжжКЯГЩТЗЯпЃК
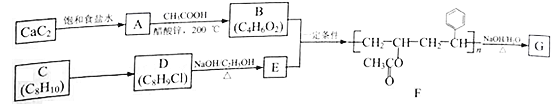
ЛиД№вдЯТЮЪЬтЃК
(1)гЩCaC2жЦБИAЕФЛЏбЇЗНГЬЪНЮЊ_________________________________ЁЃ
(2)AЩњГЩBЕФЗДгІРраЭЮЊ______________________ЁЃ
(3)CЕФЛЏбЇУћГЦЪЧ___________ЃЌCЁњDЫљашЪдМСКЭЬѕМўЗжБ№ЪЧ___________ЁЂ___________ЁЃ
(4)EЕФНсЙЙМђЪНЮЊ______________________ЁЃ
(5)ЗМЯузхЛЏКЯЮяHЪЧDЕФЭЌЗжвьЙЙЬхЃЌдђHПЩФмЕФНсЙЙЙВга___________жж(ВЛАќРЈD)ЃЌаДГіКЫДХЙВеёЧтЦзгаШ§зщЗхЧвЗхУцЛ§жЎБШЮЊ1ЉU2ЉU6ЕФHЕФЭЌЗжвьЙЙЬхЕФНсЙЙМђЪН___________(ШЮаДвЛжж)ЁЃ
(6)гЩFЩњГЩGЕФЛЏбЇЗНГЬЪНЮЊ_________________________________ЁЃ
(7)ВЮПМЬтжааХЯЂЃЌЩшМЦгЩ1ЃЌ2ЖўТШввЭщКЭБљДзЫсЮЊдСЯжЦШЁ![]() ЕФКЯГЩЯпТЗЭМ(ЮоЛњЪдМСШЮбЁ)__________________ЁЃ
ЕФКЯГЩЯпТЗЭМ(ЮоЛњЪдМСШЮбЁ)__________________ЁЃ
вбжЊЃКЃOHгыЬМЬМЫЋМќСНЖЫЕФЬМдзгжБНгЯрСЌВЛЮШЖЈЃЌЛсздБфГЩЁЊCHOЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвЛЖЈЮТЖШЪБЃЌЯђШнЛ§ЮЊ 2 L ЕФУмБеШнЦїжаГфШывЛЖЈСПЕФ SO2(g)КЭ O2(g)ЃЌЗЂЩњЗДгІЃК2SO2(g) + O2(g) ![]() 2SO3(g) ЁїH = -196 kJ/molЁЃвЛЖЮЪБМфКѓЗДгІДяЕНЦНКтзДЬЌЃЌЗДгІЙ§ГЬжаВтЖЈЕФВПЗжЪ§ОнШчБэЫљЪОЁЃ
2SO3(g) ЁїH = -196 kJ/molЁЃвЛЖЮЪБМфКѓЗДгІДяЕНЦНКтзДЬЌЃЌЗДгІЙ§ГЬжаВтЖЈЕФВПЗжЪ§ОнШчБэЫљЪОЁЃ
ЗДгІЪБМф/min | n(SO2)/mol | n(O2)/mol |
0 | 2 | 1 |
5 | 1.2 | |
10 | 0.4 | |
15 | 0.8 |
ЯТСаЫЕЗЈВЛе§ШЗЕФЪЧ
A. ЧА5 minЕФЦНОљЗДгІЫйТЪЮЊІд(SO2) = 0.08 mol/(LЁЄmin)
B. БЃГжЮТЖШВЛБфЃЌЯђЦНКтКѓЕФШнЦїжадйГфШы 0.2 mol SO2(g)КЭ0.2 mol SO3(g)ЪБЃЌІде§> ІдФц
C. ЯрЭЌЮТЖШЯТЃЌЦ№ЪМЪБЯђШнЦїжаГфШы 1.5 mol SO3(g)ЃЌДяЕНЦНКтзДЬЌЪБ SO3 ЕФзЊЛЏТЪЮЊ40%
D. БЃГжЦфЫћЬѕМўВЛБфЃЌШєЦ№ЪМЪБЯђШнЦїжаГфШы 2 mol SO3(g)ЃЌДяЕНЦНКтзДЬЌЪБЮќЪе 78.4 kJ ЕФШШСП
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com