
����Ŀ����������������Ԫ�أ����ܻ�������Ϊ���ϣ������ƾõ���ʷ���ڻ�е���졢���Բ��ϵ�����Ҳ���й㷺��Ӧ�ã���ش���������:
��1��Co��̬ԭ�ӵĵ����Ų�ʽΪ__________________________��
��2��̪ݼ�ܽ������ڹ����ϡ������Թ�ѧ���ϡ����ѧ�еĹ������������ȷ���õ��㷺��Ӧ�ã���ṹ��ͼ��ʾ����������Ϊ�����ӡ�
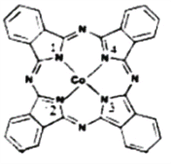
��̪ݼ�������ַǽ���ԭ�ӵĵ縺���д�С��˳��Ϊ____________��(����Ӧ��Ԫ�ط�������)��̼ԭ�ӵ��ӻ��������Ϊ___________________________��
����������ͨ����λ����ϵĵ�ԭ�ӵı����___________________________��
��3����KCN������Co2+������Һ���к�ɫ��Co(CN)2�������������ڹ�����KCN��Һ��������ɫ��[Co(CN)6]4-�����������е���λ��Ϊ________����λԭ��Ϊ____________________��
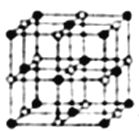
��4��Co��һ��������ľ�����ͼ��ʾ���ڸþ�������һ����ԭ�ӵȾ������������ԭ����_____������һ����ԭ�ӵȾ����Ҵν�����ԭ����______���������ܵ������ᄃ������ԭ�����������ڵ���ԭ��֮��ľ���Ϊr������ԭ��������ν��ڵ���ԭ��֮��ľ���Ϊ______����֪�ڸ��ܵ������ᄃ������ԭ�ӵİ뾶Ϊapm����ԭ�ӵİ뾶Ϊbpm�������ھ������ǽ��ܽӴ��ģ����ڸ��ܵ������ᄃ����ԭ�ӵĿռ�������Ϊ____(�ú�a��b��ʽ�ӱ�ʾ)��
��5���������Ͽ�ѧ����ʵ����һ������С�鷢������5K�³��ֳ����Եľ��壬�þ������CoO2�IJ�״�ṹ(����ͼ��ʾ��С���ʾCoԭ�ӣ������ʾOԭ��)�������ô��������ظ��ṹ��Ԫʾ��ͼ��������CoO2�Ļ�ѧ��ɵ���_______��

���𰸡� 1s22s22p63s23p63d74s2 N>C>H sp2 2��4 CN- N 12 8 ![]() r 2��/3��(a2+b2)/(a+b)3 D
r 2��/3��(a2+b2)/(a+b)3 D
����������1��CoΪ27��Ԫ�������������Ϊ27�������������ԭ�������������Ų�ʽΪ��1s22s22p63s23p63d74s2 ����ȷ���� 1s22s22p63s23p63d74s2��
��2����̪ݼ�������ַǽ���ԭ��ΪC��N��H��ͬ���ڴ����ҵ縺�����ǽ�����Խǿ�����Ե縺��N>C>H��������̼ԭ�Ӿ��γ�3��������û�й¶Ե������ӻ������Ϊ3��̼ԭ�ӵ��ӻ����Ϊsp2�ӻ�����ȷ�𰸣�N>C>H �� sp2��
�ں��йµ��Ӷ�N��Coͨ����λ������γ���λ�����γ�4�Թ��õ��Ӷԣ�1�š�3��Nԭ���γ�3�Թ��õ��Ӷ�Ϊ��ͨ�Ĺ��ۼ���2�š�4��Nԭ���γ�4�Թ��õ��Ӷԣ���Coԭ��ͨ����λ����ϣ���ȷ�𰸣�2��4��
��3��ͨ�������֪���������е���λ��ΪCN-���γ���λ����ԭ��ΪN����ȷ����CN-��N��
��4�������ɫ������ԭ�����Զ�����ԭ��Ϊ�о���������֮�������ԭ��λ��������ÿ������8���������ã�ÿ������Ϊ2���������ã��ڸþ�������һ����ԭ�ӵȾ������������ԭ�ӵĸ���Ϊ3��8/2=12����һ����ԭ�ӵȾ����Ҵν�����ԭ����8����������������ν��ڵ�������֮�����Ϊ������Խ��ߵ�һ�룬����Ϊ ![]() r���ܵ������ᄃ���й�������ԭ��8��1/8+6��1/2=4����������ԭ��12��1/4+1=4���������к���4��CoO�����Ϊ4/3������a2+b2)��4�������ı߳�Ϊ(2a+2b)�����������Ϊ(2a+2b)3���ܵ������ᄃ����ԭ�ӵĿռ�������Ϊ[4/3������a2+b2)��4]/(2a+2b)3=2��/3��(a2+b2)/(a+b)3����ȷ����12�� 8 ��
r���ܵ������ᄃ���й�������ԭ��8��1/8+6��1/2=4����������ԭ��12��1/4+1=4���������к���4��CoO�����Ϊ4/3������a2+b2)��4�������ı߳�Ϊ(2a+2b)�����������Ϊ(2a+2b)3���ܵ������ᄃ����ԭ�ӵĿռ�������Ϊ[4/3������a2+b2)��4]/(2a+2b)3=2��/3��(a2+b2)/(a+b)3����ȷ����12�� 8 �� ![]() r ��(2��/3��(a2+b2)/(a+b)3��
r ��(2��/3��(a2+b2)/(a+b)3��
��5��CoO2�ظ��ṹ��Ԫ����ԭ������ԭ����Ŀ֮����Ϊ1:2��������ͼ���֪��A����ԭ������ԭ��=1:��4��1/2��=1:2�����ϣ�B����ԭ������ԭ��=��1+4��1/4��:4=1:2�����ϣ�C����ԭ������ԭ��=��4��1/4��:��4��1/2��=1:2�����ϣ�D������ԭ������ԭ��=1����4��1/4��=1:1�������ϣ���ȷѡ��D��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и�����Ӧ���������ڹ�ҵ��������
A. �������̺�Ũ���Ṳ�������� B. ���ջ�����FeS2����SO2
C. ������ʯ���鷴Ӧ��Ư�� D. ��������Ȼ�þ�ƽ���þ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����е��뷽��ʽ��ȷ���ǣ� ��
A.H2SO4=2H++S6++4O2-B.NaHCO3=Na++H++CO32-
C.Ba(OH)2=Ba2++2OH-D.Na2CO3=Na2++CO32-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ҹ������̫���ܹ���-��ѧ��Ϸֽ������о��л���½�չ�����װ����ͼ��ʾ������˵����ȷ����

A. ��������й�������ת��Ϊ��ѧ��
B. ��װ�ù���ʱ��H+��b��������a����
C. a���Ϸ����ĵ缫��ӦΪFe3++e-=Fe2+
D. a�����費�ϲ��京Fe3+��Fe2+����Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����(Te)Ϊ��VIAԪ�أ��䵥��ƾ�����������ܳ�Ϊ�����Ͻ����Ӽ����뵼�塢���Ԫ����������ϣ������㷺Ӧ����ұ�𡢺��պ��졢���ӵ����ɴӾ���ͭ��������(��Ҫ�ɷ�ΪCu2Te)�л����ڣ�
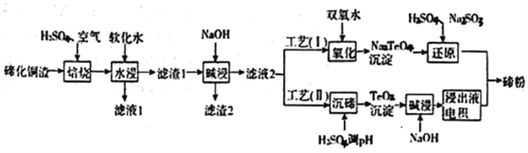
��1������������,ȷ��Ҫ��TeO2��ʽ���ڣ�д����Ӧ��Ӧ�����ӷ���ʽ:________________________��
��2��Ϊ��ѡ����ѵ����չ��ս������¶Ⱥ�������������������飬������±���ʾ:
�¶�/�� | ���������(����������) | ������/% | |
Cu | Te | ||
450 | 1.25 | 77.3 | 2.63 |
460 | 1.00 | 80.29 | 2.81 |
1.25 | 89.86 | 2.87 | |
1.50 | 92.31 | 7.70 | |
500 | 1.25 | 59.83 | 5.48 |
550 | 1.25 | 11.65 | 10.63 |
��ʵ����Ӧѡ�������Ϊ_________________��ԭ��Ϊ______________________________��
��3������1�ڼ��ʱ�����Ļ�ѧ����ʽΪ_____________________________��
��4��������I���У�����ԭ��ʱ�������ܵĻ�ѧ����ʽΪ____________________________��
��5�����ڹ�����I����������������Һ����������Ҫ��ߡ����о��߲��ù�����II����ð�.��������������У������ĵ缫��ӦʽΪ____________________________________��
��6����ҵ�����У�����2��������������Һ3������3��
����Һ3����Һ1�Ͼ�������ͭ���ϵͳ���ô�����ʩ���ŵ�Ϊ_____________________________��
������3������Auspan>��Ag������_____�����߷��롣(����ĸ)
A.��ˮ B.ϡ���� C.Ũ����������Һ D.Ũ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����:��ϩ�����Ǻ���һ������ʯ�ͻ���ˮƽ����Ҫ��־����ͼ������ϩ�ϳ������������ܵĺϳ�·�ߣ�
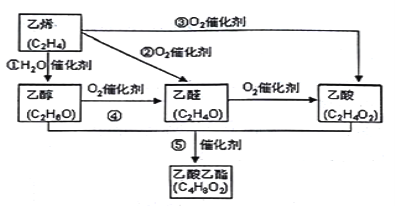
��ش��������⣺
��1����Ӧ�ܵĻ�ѧ����ʽΪ_____________________________ ��
��2����ʵ�����Ʊ���������ʱ���õ����͵�̼������Һ���������ǣ�___________________��
��3���Ҵ��ĽṹʽΪ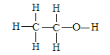 ����ʵ��֤���Ҵ�������һ��������ԭ�ӵķ�Ӧ�Ļ�ѧ����ʽΪ _________________________________��
����ʵ��֤���Ҵ�������һ��������ԭ�ӵķ�Ӧ�Ļ�ѧ����ʽΪ _________________________________��
�������к������ᣬ�����ڳ��³�ѹ����һ����ɫ��ճ�Ⱥܴ��Һ�塣ȡ9.0g������������Na��Ӧ���ڱ�״���¿��ռ���2.24L���壻��ȡ9.0g������������NaHCO3��Һ��Ӧ�����ɵ�CO2�����ڱ�״�������Ϊ2.24L����֪��������к���һ��������ش��������⣺
��1���������Է�������Ϊ��_______________________��
��2����Ũ������ڵ������£��������������Ӧ���ɻ�״��������д���÷�Ӧ�Ļ�ѧ��Ӧ����ʽ��________________________���䷴Ӧ����Ϊ��_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��E�����ֶ�����Ԫ�ء���֪�����ǵ�ԭ��������������A��Ԫ�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�Bԭ���������������������������2��C��E������Ԫ�أ�D��E��ԭ������֮��Ϊ30����D������������������ȡ��ס��ҡ������������������γɵĻ�������м����к���18�����ӡ�
������� | �� | �� | �� | �� |
�������и�Ԫ�� ԭ�Ӹ����� | A��C 1:1 | B��A 1:4 | D��E 1:3 | B��E 1:4 |
��ش��������⣺
��1��Ԫ��E�����ڱ��е�λ��Ϊ___________________________��
��2����D�ĵ��ʷŵ�NaOH��Һ�У���Ӧ�Ļ�ѧ����ʽΪ��_______________________��
��3���õ���ʽ��ʾ���γɹ��̣�_________________________��
��4�����ܱ������г���BC2��BC���ҵĻ�����干mg������������Na2O2,����������õ��ȼ����Ӧ��ȫ����ù�����������mg����BC2���ҵ������Ϊ________________��
��5����200mL MgCl2�ͱ��Ļ����Һ������c(Mg2+)�� 0.2 mol�� L-1��c(Cl-)�� 1.3mol��L-1��ҪʹMg2��ȫ��ת��Ϊ�������������������Ҫ4 mol��L-1 NaOH ��Һ������ǣ�______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͬ��ͬѹ�£���ͬ��������������ռ�е����������
A. H2 B. O2 C. CH4 D. CO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ�ϳɰ���Ӧ��N2+3H2 ![]() 2NH3��һ�����ȵĿ��淴Ӧ����Ӧ�����Ǹ��¡���ѹ��������Ҫ���ʵĴ�������֪�γ�1 mol H��H����1 mol N��H����N��N1mol���ų��������ֱ�Ϊ436 kJ��391 kJ��946 kJ����:
2NH3��һ�����ȵĿ��淴Ӧ����Ӧ�����Ǹ��¡���ѹ��������Ҫ���ʵĴ�������֪�γ�1 mol H��H����1 mol N��H����N��N1mol���ų��������ֱ�Ϊ436 kJ��391 kJ��946 kJ����:
��1����1 mol N2��ȫ��Ӧ����NH3��___(����ա��ų���)����___kJ��
��2�������1 mol N2��3 mol H2��ϣ�ʹ���ַ�Ӧ���ų���������С��������ֵ����ԭ����_______________________��
��3��ʵ����ģ�ҵ�ϳɰ�ʱ�����ݻ�Ϊ2L���ܱ�������,��Ӧ����10 min������10 mol NH3������N2��ʾ�Ļ�ѧ��Ӧ����Ϊ_____mol/(L��min)��
��4��һ�������£����ϳɰ���Ӧ�ﵽ��ѧƽ��ʱ������˵����ȷ����________
A.����Ӧ���ʺ��淴Ӧ������� B.����Ӧ�������,�淴Ӧ����Ϊ0
C.N2��ת���ʴﵽ���ֵ D.N2��H2��Ũ�����
E.N2��H2��NH3������������ F.��Ӧ�ﵽ�����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com