
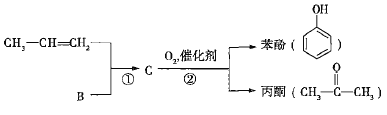
����Ŀ��ú��ʯ���ǻ���ԭ�ϵ���Ҫ��Դ����ʯ���л��A���ǹ�ҵ������A����Ҫ;����A������ֲ���������ڼ�����ú�����пɻ����B��B��̼��������Ԫ�ص�������Ϊ12��1����B�dz������л��ܼ�����ҵ�Ͽ���ͨ������;�����A��B��
![]()
![]()
(1)��ú�õ�ú���͵ķ�����Ϊ________����ʯ���ͻ��A�ķ�����Ϊ________��
(2)A��B�У���ʹ���Ը��������Һ��ɫ����________���A����B������
(3)д��B����ȡ����Ӧ�Ļ�ѧ����ʽ��________________________��дһ�����ɣ���ע����Ӧ��������
(4)��B�ͱ�ϩ��������������Ҫ�Ļ���ԭ�ϣ����ӣ�![]() ���ͱ�ͪ��
���ͱ�ͪ��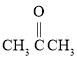 ���������������£�
���������������£�
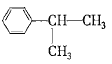
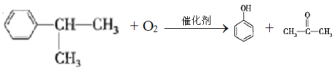
��֪��Ӧ���Ǽӳɷ�Ӧ��C��һ�ȴ�����5�֣���C�Ľṹ��ʽΪ_____________����Ӧ�ڵĻ�ѧ����ʽΪ_________________________________��
���𰸡����� �ѽ� A ![]() (��
(��![]() )
) 
��������
��ʯ���л��A���ǹ�ҵ������A����Ҫ;����A������ֲ���������ڼ�����AΪ��ϩ����ú�����пɻ����B��B��̼��������Ԫ�ص�������Ϊ12��1��![]() ����B�dz������л��ܼ�����BΪ����
����B�dz������л��ܼ�����BΪ����
(1)A����ϩ����ú�õ�ú���͵ķ�����Ϊ������ʯ���ͻ��A�ķ�ӦΪ�ѽⷴӦ���ʴ�Ϊ�������ѽ⡣
(2)A����ϩ��B�DZ�����ϩ��ʹ���Ը��������Һ��ɫ���ʴ�Ϊ��A��
(3)�������Ũ����ɷ���ȡ����Ӧ����![]() (��
(��![]() )���ʴ�Ϊ��
)���ʴ�Ϊ��![]() (��
(��![]() )��
)��
(4)��֪��Ӧ���Ǽӳɷ�Ӧ��C��һ�ȴ�����5�֣�����C��������Ӧ�õ��IJ�������õ�C�Ľṹ��ʽΪ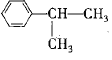 �����л���ɱ�������Ϊ���Ӻͱ�ͪ����˷�Ӧ�ڵĻ�ѧ����ʽΪ
�����л���ɱ�������Ϊ���Ӻͱ�ͪ����˷�Ӧ�ڵĻ�ѧ����ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��̼���仯������������;���ش��������⣺
��̼Ԫ����12C��13C��14C�Ⱥ��ء����ж�12C��̬ԭ�ӽṹ�ı�ʾ�����У��Ե����˶�״̬�������꾡����_______(����)��
A. B��
B��![]()
C.1s22s22p2 D��
��̬13Cԭ�Ӻ�����________�ֲ�ͬ�ռ��˶�״̬�ĵ��ӡ�
��K3[Fe(CN)6]������Fe3+��CN��֮��Ļ�ѧ��������Ϊ________���û�ѧ���ܹ��γɵ�ԭ����________��
���л��� ��________(���������������Ǽ�����)���ӣ����л����д���sp3�ӻ���ԭ�ӣ����ӦԪ�صĵ�һ�������ɴ�С��˳��Ϊ________��
��________(���������������Ǽ�����)���ӣ����л����д���sp3�ӻ���ԭ�ӣ����ӦԪ�صĵ�һ�������ɴ�С��˳��Ϊ________��
���Ҷ���(H2NCH2CH2NH2)�����װ�[N(CH3)3]�����ڰ���������Է��������ӽ������Ҷ��������װ��ķе�ߵö࣬ԭ����________��
��̼�����е������Ӳ�ͬ���ȷֽ��¶ȾͲ�ͬ���±�Ϊ����̼���ε��ȷֽ��¶Ⱥͽ��������Ӱ뾶��
̼���� | MgCO3 | CaCO3 | SrCO3 | BaCO3 |
�ȷֽ��¶�/�� | 402 | 900 | 1172 | 1360 |
���������Ӱ뾶/pm | 66 | 99 | 112 | 135 |
���Ž��������Ӱ뾶������̼���ε��ȷֽ��¶������ߣ�ԭ����________��
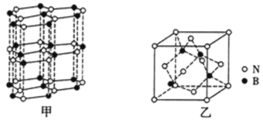
��ʯī�ľ���ṹ�;����ṹ��ͼ��ʾ����֪ʯī���ܶ�Ϊ��gcm-3��C��C����Ϊ�� cm�������ӵ�������ֵΪNA����ʯī����IJ���Ϊ________cm��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ���ҳ����Ҵ���Ũ���Ṳ����ȡ��ϩ��![]()
![]()
![]() ��ijͬѧ����ͼװ�ý�����ϩ��ȡʵ�顣������������ȷ����( )
��ijͬѧ����ͼװ�ý�����ϩ��ȡʵ�顣������������ȷ����( )
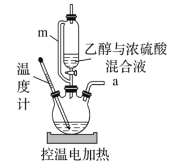
A.m��������ȷ���Ҵ���Ũ������˳������
B.�����ֵ��������165~175��
C.��Ӧ���Һ�ļ���˳������Ϊ�Ҵ���Ũ����
D.����ʱ����������ƿ��δ�����Ƭ��Ӧ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������Ҫ0.3 mol/L NaOH��Һ480 mL��1.0 mol/L������Һ250 mL��������������Һ����������ش��������⡣
��1����ͼ��ʾ��������������Һ�϶�����Ҫ����________(�����)������������Һ�����õ��IJ���������________(����������)��
��2�����ݼ�����������ƽ��ȡNaOH������Ϊ________g����ʵ����������������ȷ��������ʱ���ӿ̶��ߣ���������ҺŨ��________0.3 mol/L(����ڡ������ڡ���С�ڡ�����ͬ)����NaOH��Һ��ת��������ƿʱ����������������������ҺŨ��________0.3 mol/L��
��3�����ݼ����֪��������������Ϊ98%���ܶ�Ϊ1.84 g/cm3��Ũ��������Ϊ_______mL(����������һλС��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����֪�����Ļ�ѧʽ��NH3����Ħ������Ϊ____��6.8g NH3�����ʵ���Ϊ___mol�����״���µ����Ϊ___L���京NH3���ӵĸ���Ϊ___��������ԭ�ӵĸ���Ϊ_____��
��2���ڢ١��ڡ��ۡ��ܴ��ĺ����������ʵ������ݡ�______��__________��______��__________
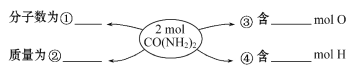
��3��12.4 g Na2R��0.4 mol Na+����Na2R��Ħ������Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��B��N��Co��Ϊ���Ͳ��ϵ���Ҫ���Ԫ�أ���ش��������⣺
�Ż�̬Coԭ�Ӻ������ռ��________�ֲ�ͬ���ܼ���������________��δ�ɶԵ��ӡ�
��Co���γ�[Co(CNO)6]3��
��1 mol�������к�����������ĿΪ________��
����CNO����Ϊ�ȵ�����ķ���Ϊ________(�ѧʽ����дһ��)��
����ͬѹǿ�£�CO������۵����N2�����ԭ����________��
�Ƿ������(NH4BF4)��������ͭ�����Ͻ����ۼ���þ�������������Ӽ�����ȼ����ũ��ɱ�桢ɱ��������֬����ȣ��Ǻϳɵ��������ܵ�ԭ��֮һ��
��1 mol NH4BF4����_______ mol�����
�ڵ�һ�����ܴ�С����B��N֮��Ļ�̬ԭ�ӵ�һ�������ɴ�С��˳��Ϊ________(��Ԫ�ط��ű�ʾ)��
��BF4���Ŀռ����幹��Ϊ________��������ԭ�ӵ��ӻ��������Ϊ________��
��BN�����ж��ֽṹ�����������൪����![]() ��ͼ��
��ͼ��![]() ��ͨ�����ڵ��ȶ��࣬��ṹ��ʯī����ȴ�����磬ԭ����_______����������������н��ʯ�ͽṹ���侧����ͼ����ʾ������������Ϊa pm�������ܶ�Ϊd gcm-3�����ӵ�������ֵ�ɱ�ʾΪ________mol-1��
��ͨ�����ڵ��ȶ��࣬��ṹ��ʯī����ȴ�����磬ԭ����_______����������������н��ʯ�ͽṹ���侧����ͼ����ʾ������������Ϊa pm�������ܶ�Ϊd gcm-3�����ӵ�������ֵ�ɱ�ʾΪ________mol-1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����˾ƥ��(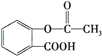 )�dz��õĽ�����ʹҩ������˵������ȷ����( )
)�dz��õĽ�����ʹҩ������˵������ȷ����( )
A.1mol��˾ƥ�ֿ�����3molNaOH��Ӧ
B.��˾ƥ����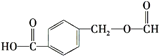 ��Ϊͬ���칹��
��Ϊͬ���칹��
C.��˾ƥ�ֿ��Է���ȡ����Ӧ���ӳɷ�Ӧ��������Ӧ
D.1mol��˾ƥ�ֿ�����5molH2�����ӳɷ�Ӧ��Ҳ����1mol̼�����Ʒ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ѪҺ����![]() ��
��![]() �Ȼ���ԡ������£�ˮ��Һ�и�����Ե���Ũ��֮�ȵĶ���ֵ
�Ȼ���ԡ������£�ˮ��Һ�и�����Ե���Ũ��֮�ȵĶ���ֵ![]() ��ʾ
��ʾ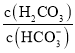 ��
��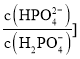 ��pH�Ĺ�ϵ��ͼ��ʾ����֪̼��
��pH�Ĺ�ϵ��ͼ��ʾ����֪̼��![]() ������
������![]()
![]() ��������˵������ȷ���ǣ� ��
��������˵������ȷ���ǣ� ��
A.���ߢ��ʾ ��pH�ı仯��ϵ
��pH�ı仯��ϵ
B.![]() �Ĺ����У�ˮ�ĵ���̶�������
�Ĺ����У�ˮ�ĵ���̶�������
C.��![]()
![]() ʱ��
ʱ��![]()
D.��pH����ʱ��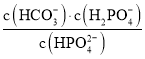 ������
������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������������жϣ�����˵����ȷ���ǣ� ��
A.����ˮ�����Եģ������Ȳ����ᣬҲ���Ǽ�
B.![]() ���Ա���������������ʣ���
���Ա���������������ʣ���![]() ���ƣ�
���ƣ�![]() Ҳ���Ա����������������
Ҳ���Ա����������������
C.![]() ��Һ�����ԣ�
��Һ�����ԣ�![]() ֻ�ܱ�������
ֻ�ܱ�������
D.������ͼ��������������������������κ�ˮҲ�����������������������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com