
����Ŀ��ʵ�����Ʊ����״��ͱ�����Ļ�ѧԭ����
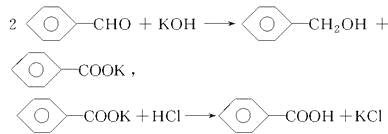
��֪����ȩ�ױ��������������״��ķе�Ϊ205.3 ������������۵�Ϊ121.7 �����е�Ϊ249 �����ܽ��Ϊ0.34 g�����ѵķе�Ϊ34.8 ����������ˮ���Ʊ����״��ͱ��������Ҫ����������ʾ��
�Ը���������Ϣ���ж�����˵���������(����)
A. ����������ȡ��Һ
B. ������Һ�����ܽ����Ҫ�ɷ��DZ��״�
C. �������������ò�Ʒ���DZ��״�
D. ���������˵õ���Ʒ���DZ������
���𰸡�D
�����������������A���ӹ����Ͽ��������õ�������Һ��ˮ��Һ���������ǻ������ܵ�Һ�壬������ȡ�ͷ�Һ�ķ�������ȷ��B������ȩ���������������ɱ��Ҵ��ͱ�����أ������Ʊ�������ͱ��Ҵ����Ʊ����̣�������Һ�ܽ����Ҫ�ɷ��DZ��״�����ȷ��C�������������˱��״��ķе�Ϊ205.3�������ѵķе�Ϊ34.8���������ܣ����÷е㲻ͬ����������ķ����õ����Ѻͱ��Ҵ�����ȷ��D��������ˮ��Һ�м������ᣬ�������ת��ɱ����ᣬ���������ˮ�����ù��˵ķ������з��룬����


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ��Ч������������������ǿ�Ƹ�ȼú��ҵҪ��ȼú�����������������������ŷš��ش���������:
(1)ȼú���������������漰���IJ��ַ�Ӧ����:
a.4NH3(g)+6NO(g) ![]() 5N2(g)+6H2O(g) ��H 1
5N2(g)+6H2O(g) ��H 1
b.4NH3(g)+5O2(g) ![]() 4NO(g)+6 H2O(g)��H2 =-925kJ��mol-1
4NO(g)+6 H2O(g)��H2 =-925kJ��mol-1
c.N2(g)+O2(g) ![]() 2NO(g)��H 3=+175kJ��mol-1
2NO(g)��H 3=+175kJ��mol-1
���H 1=_____kJ/mol��
(2)��ij�����ܱ������м���2molNH3��3molNO�����ʵ������·���(1)�з�Ӧa����Ӧ������NO��ƽ��ת�������¶�T��ѹǿp�ı仯������ͼ��ʾ:
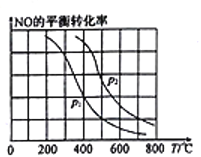
��p1________p2(�>����<����=��)��
������ѹǿΪ��p2���¶�Ϊ600��ʱ���ﵽƽ��ʱ��Ӧ��ϵ�ڻ�ѧ�ܽ�����300kJ����NO��ת����Ϊ_______________________��
��600��ʱ�����и����������ܱ����÷�Ӧ�Ѵﵽ��ѧƽ��״̬����______��
a.ˮ��NO������������� b.���������ܶȱ��ֲ���
c. NH3��NO�������ʱ�Ϊ5:4 d.��������ѹǿ���ֲ���
(3)��2molN2��3molH2O��0.5molNO�Ļ����������x��y��z�����ݻ���ͬ�ĺ����ܱ������У������ʵ�������������Ӧ����Ӧ������c(NO)��ʱ��ı仯��ͼ��ʾ��
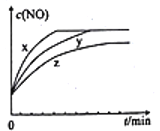
���ڽ���ƽ��Ĺ����У����������з�Ӧ���ʵ���Դ�СΪ______________��
����y�����з�Ӧ��ȣ�z�����з�Ӧ�ı���������ж����ݷֱ���_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
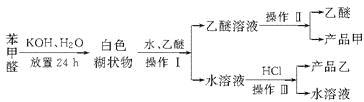
����Ŀ����1��ij�������A������ͼ��������Է�������Ϊ84��������ױ��������к���̼̼˫�����˴Ź������ױ���������ֻ��һ�����͵��⡣��A�Ľṹ��ʽ��_____________��
��2����Ⱥͺ��������ϸ����Ķ�Ʒ����ȷ�����C��H��N��O�����������ֱ�Ϊ71.58%��6.67%��4.91%��16.84%����֪����Է���������200~300֮�䡣��ȵ���Է�������Ϊ_____________����ȵĻ�ѧʽΪ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ij�л���Ľṹ��
��1���ⶨʵ��ʽ��ij��̼���⡢������Ԫ�ص��л����ȼ�շ���ʵ��ⶨ��̼������������64.86%���������������13.51%������ʵ��ʽ��________��
��2��ȷ������ʽ����ͼ�Ǹ��л��������ͼ��������Է�������Ϊ______������ʽΪ________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����º������ò����������Ƶ������������д�����ϸ��,�����á�ӫ���ء���ӫ����ø����ⷨ�����г������⺬ϸ�����ٽ��м��:�ٽ�������ĥ�����Ĵ���,ȡһ��������Һ����ֹ��ȼ�(�ⶨ����ǿ�ȵ�����)��Ӧ����,����������ӫ���غ�ӫ����ø,�����������½��з�Ӧ;�ڼ�¼����ǿ�Ȳ�����ATP����;�۲����ϸ���������������ش���������:
(1)ӫ���ؽ���____�ṩ��������ͱ�����,��ӫ����ø���������γ�����ӫ���ز��ҷ���ӫ�⡣���ݷ���ǿ�ȿ��Լ����������֯��ATP�ĺ���,ԭ���Ƿ���ǿ����ATP������____________(����/����);����ATP�������������ϸ��������������:ÿ��ϸ��ϸ����ATP����__________��
(2)��ӫ���ء���ӫ����ø����ⷨ�����漰������ת����_________;����ϸ����ATP��ˮ��һ����________(���ܷ�Ӧ����ܷ�Ӧ)����ϵ��
(3)�о���Ա�ò�ͬ��������ӫ����ø��,�ⶨøŨ���뷢��ǿ����ͼ��ʾ��
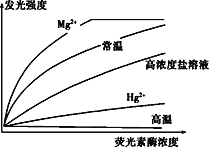
���и�Ũ������Һ��ϡ�ͺ�ø���Կ��Իָ�,���º�Hg2+������ø���Բ��ɻָ�����Ҫ��ʡӫ����ø������,����ʹ��____����;Hg2+������ø���Խ��Ϳ�������Ϊ______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����I��Ϊ��֤��NH3��H2O��������ʣ��ס��ҡ������˷ֱ�ѡ�������Լ�����ʵ�顣0.010mol��L��1��ˮ��0.1mol��L��1NH4Cl��Һ��NH4Cl���塢��̪��Һ��pH��ֽ������ˮ��
�ټ���pH��ֽ���0.010mol��L��1��ˮ��pHΪ10�����϶�NH3��H2O��������ʣ�����Ϊ��һ����________(���ȷ������ȷ��)����˵�����ɣ�__________________________________��
����ȡ��10mL0.010mol��L��1��ˮ����pH��ֽ����pH��a��Ȼ��������ˮϡ����1000mL������pH��ֽ����pH��b����Ҫȷ��NH3��H2O��������ʣ���a��bֵӦ����Ĺ�ϵ��_____��
(II)(1)�����£���pH��6��CH3COOH��CH3COONa�Ļ����Һ��ˮ���������c(OH��)��________��
(2)������ˮ��Һ��Ϊ��ˮ�����д��ڵ���Ҫ��������NH3��H2O��
��֪��a.�����£������NH3��H2O�ĵ���ƽ�ⳣ����Ϊ1.74��10��5��
b.CH3COOH��NaHCO3===CH3COONa��CO2����H2O��
��CH3COONH4��Һ��________��(��ᡱ��������С�����ͬ)��NH4HCO3��Һ��________�ԣ�NH4HCO3��Һ�����ʵ���Ũ������������________(�ѧʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ���ҳ����ü�ȩ���ⶨ(NH4)2SO4��Ʒ�е��������������䷴Ӧԭ��Ϊ��4NH4��+6HCHO=3H��+6H2O+(CH2)6N4H�� ��Ȼ����NaOH����Һ�ζ���Ӧ���ɵ��ᣬij��ȤС���ü�ȩ������������ʵ�飺
����I ��ȡ��Ʒ1.500g��
����II ����Ʒ�ܽ����ȫת�Ƶ�250 mL����ƿ�У����ݣ����ҡ�ȡ�
����� ��ȡ25.00mL��Ʒ��Һ��250mL��ƿ�У�����10mL20�������Լ�ȩ��Һ��ҡ�ȡ�����5 min��,����1~2�η�̪��Һ����NaOH����Һ�ζ����յ㡣�����������������ظ�2�Ρ�
(1)���ݲ������գ�
�ٵ���ζ��յ�ʱ�����Ӽ�ʽ�ζ��ܶ�����������Ʒ�е�����������________(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��
�ڴﵽ�ζ��յ�ʱ�����ּ�ʽ�ζ��ܵļ�������ݣ���ζ�ʱ��ȥNaOH����Һ�����__________ (�ƫ����ƫС������Ӱ�족)
�۵ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲�___________
�ܵζ��ﵽ�յ��жϣ�__________________________________________
(2)�ζ�������±���ʾ��
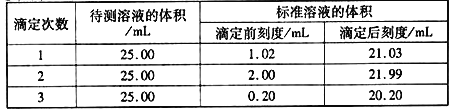
��NaOH����Һ��Ũ��Ϊ0.1010mol��L��1�����Ʒ�е�����������Ϊ______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼΪ���ú�ˮ�Ʊ��������ʵĹ��̡������й�˵����ȷ����
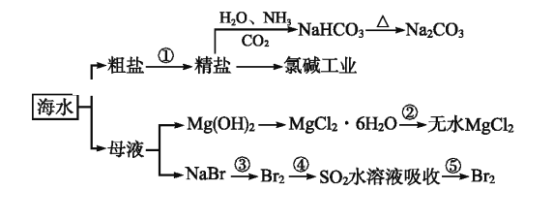
A. ��ȡNaHCO3�ķ�Ӧ���������ܽ��С��NaCl���ܽ��
B. �ó����ʯ��ˮ�ɼ���NaHCO3��Һ��Na2CO3��Һ
C. �ڵڢۡ��ܡ��ݲ�����,��Ԫ�ؾ�������
D. ����MgCl2��Һ��ȡ��ˮ�Ȼ�þ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������SO2��CO��NOx��Ⱦ�ǻ�ѧ�������о�����Ҫ���⡣
��.���᳧�����ŷź�SO2��β����Ի����������Σ����
��1����ҵ�Ͽ����÷ϼ�Һ����Ҫ�ɷ�ΪNa2CO3���������᳧β���е�SO2���õ�Na2SO3��Һ���÷�Ӧ�����ӷ���ʽΪ__________________________________��
��.�������������Ϊ��Ӧ��2CO(g)+O2(g)![]() 2CO2(g)�Ĵ�����ͼ�ױ�ʾ����ͬ�ĺ����ܱ���������ͬ��ʼŨ�ȡ���ͬ��Ӧʱ�����,ʹ��ͬ�����IJ�ͬ��������������͡����ͣ���ʱ��CO��ת�������¶ȵĹ�ϵ��
2CO2(g)�Ĵ�����ͼ�ױ�ʾ����ͬ�ĺ����ܱ���������ͬ��ʼŨ�ȡ���ͬ��Ӧʱ�����,ʹ��ͬ�����IJ�ͬ��������������͡����ͣ���ʱ��CO��ת�������¶ȵĹ�ϵ��

��2��a��b��c��d �ĵ��У��ﵽƽ��״̬����__________________________________��
��3����֪c��ʱ������O2Ũ��Ϊ0.02 mol/L����50��ʱ���ڦ��������������COת����Ӧ��ƽ�ⳣ��K=____________���ú�x�Ĵ���ʽ��ʾ����
��4�����й���ͼ��˵����ȷ����_____________��
A.COת����Ӧ��ƽ�ⳣ��K(a)
B.�ھ�δ�ﵽƽ��״̬ʱ��ͬ���¦��������������COת�����ʱȦ���Ҫ��
C.b��ʱCO��O2����֮�䷢����Ч��ײ�ļ���������ʵ����������
D.e��ת���ʳ���ͻ���ԭ��������¶����ߺ����ʧȥ����
��.ij���ܴ������Դ��������ͳ�β���е�̼��(C)��NOx����ͬ�¶��£���ģ��β�����ɷ����±���ʾ������ͬ������ͨ���ô�����������в��CO2��N2��N2O����NO��������ݽ����ͼ����ʾ��
ģ��β�� | ����(10mol) | ̼�� | ||
NO | O2 | He | ||
���ʵ���(mol) | 0.025 | 0.5 | 9.475 | n |
��5��375��ʱ������ų��������к�0.45 molO2��0.0525 mol CO2����Y�Ļ�ѧʽΪ______________��

��6��ʵ������в���NOģ��NOx����������NO2��ԭ����____________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com