
ЁОЬтФПЁПAЁЂBЁЂCЁЂDЁЂEЁЂFСљжждЊЫиЕФдзгађЪ§вРДЮЕндіЁЃвбжЊЃКЂйFЕФдзгађЪ§ЮЊ29ЃЌЦфгрЕФОљЮЊЖЬжмЦкжїзхдЊЫиЃЛЂкEдзгМлЕчзг(ЭтЮЇЕчзг)ХХВМЮЊmsnmpnЃ1ЃЛЂлDдзгзюЭтВуЕчзгЪ§ЮЊХМЪ§ЃЛЂмAЁЂCдзгpЙьЕРЕФЕчзгЪ§ЗжБ№ЮЊ2КЭ4ЁЃ
ЧыЛиД№ЯТСаЮЪЬтЃК
(1)ЯТСаа№Ъіе§ШЗЕФЪЧ________(ЬюађКХ)ЁЃ
AЃЎН№ЪєМќЕФЧПШѕЃКDЃОE
BЃЎЛљЬЌдзгЕквЛЕчРыФмЃКDЃОE
CЃЎЮхжждЊЫижаЃЌЕчИКадзюДѓЕФдЊЫиЪЧE
DЃЎОЇИёФмЃКNaClЃМDCl2
(2)FЛљЬЌдзгЕФКЫЭтЕчзгХХВМЪНЮЊ_____ЃЛгыFЭЌвЛжмЦкЕФИБзхдЊЫиЕФЛљЬЌдзгжазюЭтВуЕчзгЪ§гыFдзгЯрЭЌЕФдЊЫиЮЊ_____(ЬюдЊЫиЗћКХ)ЁЃ
(3)EЕЅжЪОЇЬхжадзгЕФЖбЛ§ЗНЪНШчЯТЭММзЫљЪОЃЌЦфОЇАћЬиеїШчЯТЭМввЫљЪОЃЌдзгжЎМфЯрЛЅЮЛжУЙиЯЕЕФЦНУцЭМШчЯТЭМБћЫљЪОЁЃ
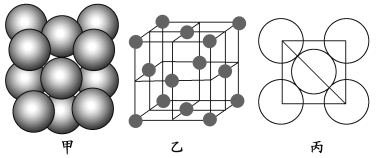
ШєвбжЊEЕФдзгАыОЖЮЊdЃЌNAДњБэАЂЗќМгЕТТоГЃЪ§ЃЌEЕФЯрЖддзгжЪСПЮЊMrЃЌдђвЛИіОЇАћжаEдзгЕФЪ§ФПЮЊ________ЃЌИУОЇЬхЕФУмЖШЮЊ_______________ (гУзжФИБэЪО)ЁЃ
ЁОД№АИЁПBD 1s22s22p63s23p63d104s1 Cr 4 Mr/(4ЁС21/2 d3NA)
ЁОНтЮіЁП
AЁЂBЁЂCЁЂDЁЂEЁЂFСљжждЊЫиЕФдзгађЪ§вРДЮЕндіЁЃГ§FдЊЫиЭтЃЌЦфгрЕФОљЮЊЖЬжмЦкжїзхдЊЫиЁЃЂйFЕФдзгађЪ§ЮЊ29ЃЌдђFЮЊЭдЊЫиЃЛЂмAЁЂCдзгpЙьЕРЕФЕчзгЪ§ЗжБ№ЮЊ2КЭ4ЃЌдђAКЫЭтЕчзгХХВМЪНЮЊ1s22s22p2ЃЌдђAЮЊЬМдЊЫиЃЛCКЫЭтЕчзгХХВМЪНЮЊ1s22s22p4ЃЌдђCЮЊбѕдЊЫиЃЌBдЊЫидзгађЪ§НщгкЬМдЊЫигыбѕдЊЫижЎМфЃЌдђBЮЊЕЊдЊЫиЃЛЂкEдзгМлЕчзг(ЭтЮЇЕчзг)ХХВМЮЊmsnmpn-1ЃЌsФмМЖШнФЩ2ИіЕчзгЃЌдђEЭтЮЇЕчзгХХВМЮЊms2mp1ЃЌдзгађЪ§ДѓгкбѕдЊЫиЃЌдђEЮЊТСдЊЫиЃЛЂлDдзгзюЭтВуЕчзгЪ§ЮЊХМЪ§ЃЌDдзгађЪ§НщгкбѕдЊЫигыТСдЊЫижЎМфЃЌдђDЮЊУОдЊЫиЁЃОнДЫНсКЯдЊЫижмЦкТЩКЭОЇАћЕФЯрЙижЊЪЖЗжЮіНтД№ЁЃ
ИљОнЩЯЪіЗжЮіЃЌAЮЊЬМдЊЫиЃЌBЮЊЕЊдЊЫиЃЌCЮЊбѕдЊЫиЃЌDЮЊУОдЊЫиЃЌEЮЊТСдЊЫиЃЌFЮЊЭдЊЫиЁЃ
(1)AЃЎDЮЊУОдЊЫиЃЌEЮЊТСдЊЫиЃЌТСРызгАыОЖаЁгкУОРызгАыОЖЃЌЧвТСЕФМлЕчзгЪ§ФПДѓгкУОЃЌЫљвдН№ЪєМќDЃМEЃЌЙЪAДэЮѓЃЛBЃЎЭЌвЛжмЦкжаЃЌдЊЫиЕФЕквЛЕчРыФмЫцзХдзгађЪ§ЕФдіДѓЖјГЪдіДѓЧїЪЦЃЌЕЋЕкЂђAзхЁЂЕкVAзхдЊЫиЕквЛЕчРыФмДѓгкЯрСкдЊЫиЃЌЫљвдDЃОEЃЌЙЪBе§ШЗЃЛCЃЎЗЧН№ЪєаддНЧПЃЌЕчИКаддНДѓЃЌЮхжждЊЫижаЃЌЕчИКадзюДѓЕФдЊЫиЪЧO(C)ЃЌЙЪCДэЮѓЃЛDЃЎРызгАыОЖФЦРызгДѓгкУОРызгЃЌЧвФЦРызгЕчКЩЪ§аЁгкУОРызгЃЌТШЛЏУОжаРызгМќИќЧПЃЌОЇИёФмЃКNaClЃМMgCl2ЃЌЙЪDе§ШЗЃЛЙЪбЁBDЃЛ
(2)FЮЊЭдЊЫиЃЌКЫФкжЪзгЪ§ЮЊ29ЃЌКЫЭтЕчзгЪ§ЮЊ29ЃЌКЫЭтЕчзгХХВМЪНЪЧ1s22s22p63s23p63d104s1ЃЛгыFЭЌвЛжмЦкЕФИБзхдЊЫиЕФЛљЬЌдзгжазюЭтВуЕчзгЪ§гыFдзгЯрЭЌЕФдЊЫиЃЌМлВуЕчзгХХВМЮЊ3d54s1ЃЌКЫЭтЕчзгХХВМЪНЪЧ1s22s22p63s23p63d54s1ЃЌЪЧCrдЊЫиЃЌЙЪД№АИЮЊЃК1s22s22p63s23p63d104s1ЃЛCrЃЛ
(3)ОЇАћжаAlдзгЪ§ФПЮЊЃК8ЁС![]() +6ЁС
+6ЁС![]() =4ЃЛИУОЇАћЕФжЪСПm=4ЁС
=4ЃЛИУОЇАћЕФжЪСПm=4ЁС![]() ЃЌИљОнБћЭМПЩжЊЃЌОЇАћЕФУцЖдНЧЯпЮЊ4dЃЌдђОЇАћЕФРтГЄ=
ЃЌИљОнБћЭМПЩжЊЃЌОЇАћЕФУцЖдНЧЯпЮЊ4dЃЌдђОЇАћЕФРтГЄ=![]() ЁС4dЃЌОЇАћЕФЬхЛ§V=(4ЁСdЁС
ЁС4dЃЌОЇАћЕФЬхЛ§V=(4ЁСdЁС![]() )3=16
)3=16![]() d3ЃЌОЇАћУмЖШІб=
d3ЃЌОЇАћУмЖШІб=![]() =
=![]() ЃЌЙЪД№АИЮЊЃК4ЃЛ
ЃЌЙЪД№АИЮЊЃК4ЃЛ![]() ЁЃ
ЁЃ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПSCRКЭNSRММЪѕПЩгааЇНЕЕЭВёгЭЗЂЖЏЛњдкПеЦјЙ§СПЬѕМўЯТЕФNOxХХЗХЁЃ
(1)SCRЃЈбЁдёадДпЛЏЛЙдЃЉЙЄзїдРэЃК
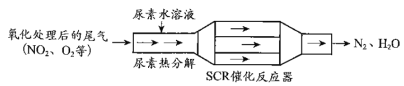
ЂйФђЫи[CO(NH2)2]ЫЎШмвКШШЗжНтЮЊNH3КЭCO2ЃЌИУЗДгІЕФЛЏбЇЗНГЬЪНЃК__________________
ЂкЗДгІЦїжаNH3ЛЙдNO2ЕФЛЏбЇЗНГЬЪНЃК________________________ЃЛ
ЂлЕБШМгЭжаКЌСђСПНЯИпЪБЃЌЮВЦјжаSO2дкO2зїгУЯТЛсаЮГЩ(NH4)2SO4ЃЌЪЙДпЛЏМСжаЖОЁЃгУЛЏбЇЗНГЬЪНБэЪО(NH4)2SO4ЕФаЮГЩЃК_____________________________ЃЛ
ЂмФђЫиШмвКХЈЖШгАЯьNO2ЕФзЊЛЏЃЌВтЖЈШмвКжаФђЫиЃЈM=60 gЁЄmol 1ЃЉКЌСПЕФЗНЗЈШчЯТЃКШЁa gФђЫиШмвКЃЌНЋЫљКЌЕЊЭъШЋзЊЛЏЮЊNH3ЃЌЫљЕУNH3гУЙ§СПЕФv1 mL c1 molЁЄL1 H2SO4ШмвКЮќЪеЭъШЋЃЌЪЃгрH2SO4гУv2 mL c2 molЁЄL1 NaOHШмвКЧЁКУжаКЭЃЌдђФђЫиШмвКжаШмжЪЕФжЪСПЗжЪ§ЪЧ_________________ЃЛ
(2)NSR(NOxДЂДцЛЙд)ЙЄзїдРэЃКNOxЕФДЂДцКЭЛЙддкВЛЭЌЪБЖЮНЛЬцНјааЃЌШчЭМaЫљЪОЁЃ
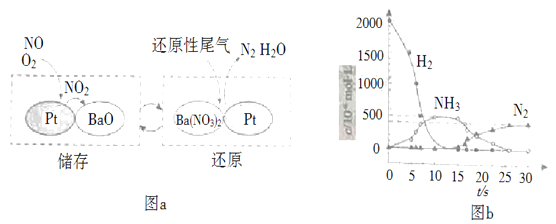
ЂйЭЈЙ§BaOКЭBa(NO3)2ЕФЯрЛЅзЊЛЏЪЕЯжNOxЕФДЂДцКЭЛЙдЁЃЛЙдNOxЕФЮяжЪЪЧ_________________ЃЛ
ЂкгУH2ФЃФтЮВЦјжаЛЙдадЦјЬхбаОПСЫBa(NO3)2ЕФДпЛЏЛЙдЙ§ГЬЃЌИУЙ§ГЬЗжСНВННјааЃЌЭМbБэЪОИУЙ§ГЬЯрЙиЮяжЪХЈЖШЫцЪБМфЕФБфЛЏЙиЯЕЁЃЕкЖўВНЗДгІЯћКФЕФNH3гыBa(NO3)2ЕФЮяжЪЕФСПжЎБШЪЧ__________________ЃЛ
ЂлЛЙдЙ§ГЬжаЃЌгаЪБЛсВњЩњаІЦјЃЈN2OЃЉЁЃгУЭЌЮЛЫиЪОзйЗЈбаОПЗЂЯжаІЦјЕФВњЩњгыNOгаЙиЁЃдкгабѕЬѕМўЯТ15NOгыNH3вдвЛЖЈБШР§ЗДгІЪБЃЌЕУЕНЕФаІЦјМИКѕЖМЪЧ15NNOЁЃНЋИУЗДгІЕФЛЏбЇЗНГЬЪНВЙГфЭъећЃК__________________![]() ____15NNO+___H2O
____15NNO+___H2O
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТЭМжаУПвЛЗНПђЕФзжФИДњБэвЛжжЗДгІЮяЛђЩњГЩЮяЃК
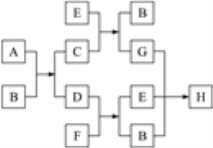
вбжЊЦјЬЌЬўD(ЦфУмЖШдкЭЌЮТЭЌбЙЯТЪЧЧтЦјУмЖШЕФ13БЖ)гыЮяжЪFЗДгІЪБВњЩњУїССЖјДјХЈСвКкбЬЕФЛ№бцЁЃ
(1)ЧыаДГіЯТСазжФИЫљДњБэЮяжЪЕФЛЏбЇЪН(ЛђЗжзгЪН)ЃКA______ЁЂH______;
(2)ЧыаДГіЯТСазжФИЫљДњБэЮяжЪЕФЕчзгЪНЃКBЃЎ____________ EЃЎ__________________
(3)ЧыаДГіЯТСазжФИЫљДњБэЮяжЪЕФНсЙЙЪНЃКDЃЎ_____________ E _________
(4)аДГіЪЕбщЪвжЦШЁDЕФЛЏбЇЗНГЬЪН: __________________
(5)ЧыаДГіD+F Ёњ E+BЕФЛЏбЇЗНГЬЪН:________________
(6)DдкДпЛЏМСВЂМгШШЬѕМўЯТПЩвдЗЂЩњМгОлЗДгІЃЌЪдаДГіИУЗДгІЕФЛЏбЇЗНГЬЪНЃК______
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТБэжаЦРМлКЯРэЕФЪЧ( )
бЁЯю | ЛЏбЇЗДгІМАЦфРызгЗНГЬЪН | ЦРМл |
A | ЬњгыЯЁбЮЫсЗДгІЃК | е§ШЗ |
B | ДѓРэЪЏШмгкДзЫсЕФЗДгІЃК | ДэЮѓЃЌДзЫсгІаДЮЊЗжзгаЮЪНCH3COOHЃЌCaCO3гІаДГЩРызгаЮЪН |
C | ЯђBa(OH)2ШмвКжаЕЮМгЩйСПNaHCO3ШмвКЃКBa2++ OH-+HCO3- = BaCO3Ё§+ H2O | ДэЮѓЃЌЗНГЬЪНжаBa2+КЭOH-ЛЏбЇМЦСПЪ§жЎБШЮЊ1ЁУ2 |
D | NH4HCO3ШмвКгыЙ§СПKOHХЈШмвКЙВШШЃК | ДэЮѓЃЌHCO3-вВПЩвдгыOHЃЗДгІ |
A.AB.BC.CD.D
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПУїЗЏЪЏЕФжївЊГЩЗжЪЧK2SO4ЁЄAl2(SO4)3ЁЄ2Al 2O3ЁЄ6H2OЃЌЛЙКЌгаЩйСПFe2O3дгжЪЁЃРћгУУїЗЏЪЏжЦБИЧтбѕЛЏТСЕФСїГЬШчЯТЃК
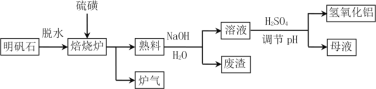
ЃЈ1ЃЉБКЩеТЏжаЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЮЊ2Al2(SO4)3ЃЋ3S ![]() 2Al 2O3 ЃЋ9SO2ЃЌИУЗДгІЕФбѕЛЏМСЪЧ______________ЃЌШєЩњГЩ1molAl2O3ЃЌдђзЊвЦЕФЕчзгЪ§ЮЊ____________________ЁЃ
2Al 2O3 ЃЋ9SO2ЃЌИУЗДгІЕФбѕЛЏМСЪЧ______________ЃЌШєЩњГЩ1molAl2O3ЃЌдђзЊвЦЕФЕчзгЪ§ЮЊ____________________ЁЃ
ЃЈ2ЃЉНЋБъПіЯТ1.12LТЏЦјЭЈШы100mL 0.5molЁЄL-1 NaOHШмвКжаЃЌЕУЕНвЛжжЫсадШмвКЃЌдђИУШмвКжаИїжжРызгХЈЖШгЩДѓЕНаЁЕФХХСаЫГађЮЊ___________________________________ЁЃ
ЃЈ3ЃЉЪьСЯШмНтЪБЗДгІЕФРызгЗНГЬЪНЮЊ_________________________ЁЃ
ЃЈ4ЃЉФИвКжаШмжЪжївЊГЩЗжЕФЛЏбЇЪНЮЊ____________ЁЂ_____________ЃЌШмвКЕїНкpHКѓОЙ§ТЫЁЂЯДЕгПЩЕУAl(OH)3ГСЕэЃЌжЄУїГСЕэвбЯДЕгИЩОЛЕФЪЕбщВйзїКЭЯжЯѓЪЧ ________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвбжЊXЁЂYКЭZШ§жждЊЫиЕФдзгађЪ§жЎКЭЕШгк42ЁЃXдЊЫидзгЕФ4pЙьЕРЩЯга3ИіЮДГЩЖдЕчзгЃЌYдЊЫидзгЕФзюЭтВу2pЙьЕРЩЯга2ИіЮДГЩЖдЕчзгЁЃXИњYПЩаЮГЩЛЏКЯЮяX2Y3ЃЌZдЊЫиПЩвдаЮГЩИКвЛМлРызгЁЃЧыЛиД№ЯТСаЮЪЬтЃК
(1)XдЊЫидзгЛљЬЌЪБЕФЕчзгХХВМЪНЮЊ____________ЃЌИУдЊЫиЕФЗћКХЪЧ________ЁЃ
(2)YдЊЫидзгЕФМлВуЕчзгЕФЙьЕРБэЪОЪНЮЊ___________ЃЌИУдЊЫиЕФУћГЦЪЧ________ЁЃ
(3)вбжЊЛЏКЯЮяX2Y3дкЯЁСђЫсШмвКжаПЩБЛН№ЪєаПЛЙдЮЊXZ3ЃЌВњЮяЛЙгаZnSO4КЭH2OЃЌИУЗДгІЕФЛЏбЇЗНГЬЪНЪЧ________ЁЃ
(4)БШНЯXЕФЧтЛЏЮягыЭЌзхЕкЖўЁЂШ§жмЦкдЊЫиЫљаЮГЩЕФЧтЛЏЮяЮШЖЈадВЂЫЕУїРэгЩ______________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПШчЭМЮЊдЊЫижмЦкБэжаЖЬжмЦкЕФвЛВПЗж,ЫФжждЊЫиОљВЛЪЧЯЁгаЦјЬхдЊЫиЁЃЯТСаЙигкетЫФжждЊЫиМАЦфЛЏКЯЮяЕФЫЕЗЈжае§ШЗЕФЪЧЃЈ ЃЉ

A. дзгАыОЖЃКW>Z
B. ЦјЬЌЧтЛЏЮяЕФЮШЖЈадЃКW>X
C. WЕФзюИпе§ЛЏКЯМлгыИКЛЏКЯМлЕФОјЖджЕПЩФмЯрЕШ
D. ZЕФзюИпМлбѕЛЏЮяЕФЫЎЛЏЮяПЩФмЮЊЧПМю
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПВЛЭЌдЊЫиЕФдзгдкЗжзгФкЮќв§ЕчзгЕФФмСІДѓаЁПЩгУвЛЖЈЪ§жЕxРДБэЪОЃЌШєxдНДѓЃЌЦфдзгЮќв§ЕчзгЕФФмСІдНЧПЃЌдкЫљаЮГЩЕФЗжзгжаГЩЮЊИКЕчКЩвЛЗНЁЃ
ЯТУцЪЧФГаЉЖЬжмЦкдЊЫиЕФxжЕЃК
дЊЫиЗћКХ | Li | Be | B | C | O | F | Na | Al | Si | P | S | Cl |
xжЕ | 0.98 | 1.57 | 2.04 | 2.55 | 3.44 | 3.98 | 0.93 | 1.61 | 1.90 | 2.19 | 2.58 | 3.16 |
ЃЈ1ЃЉЭЈЙ§ЗжЮіxжЕБфЛЏЙцТЩЃЌШЗЖЈMgЕФxжЕЗЖЮЇЃК____<x(Mg)< _____ЁЃ
ЃЈ2ЃЉЭЦВтxжЕгыдзгАыОЖЕФЙиЯЕЪЧ____ЃЛИљОнЖЬжмЦкдЊЫиЕФxжЕБфЛЏЬиЕуЃЌЬхЯжСЫдЊЫиаджЪЕФ________БфЛЏЙцТЩЁЃ
ЃЈ3ЃЉФГгаЛњЛЏКЯЮяНсЙЙЪНЮЊ![]() ЃЌЦфжаSЁЊNжаЃЌФуШЯЮЊЙВгУЕчзгЖдЦЋЯђЫЃП__ЃЈаДдзгУћГЦЃЉЁЃ
ЃЌЦфжаSЁЊNжаЃЌФуШЯЮЊЙВгУЕчзгЖдЦЋЯђЫЃП__ЃЈаДдзгУћГЦЃЉЁЃ
ЃЈ4ЃЉОбщЙцТЩИцЫпЮвУЧЃКЕБГЩМќЕФСНдзгЯргІдЊЫиЕФВюжЕЃЈІЄxЃЉЃЌЕБІЄx>1.7ЪБЃЌвЛАуЮЊРызгМќЃЌЕБІЄx<1.7ЪБЃЌвЛАуЮЊЙВМлМќЃЌЪдЭЦЖЯAlBr3жаЛЏбЇМќРраЭЪЧ______ЁЃ
ЃЈ5ЃЉдЄВтдЊЫижмЦкБэжаЃЌxжЕзюаЁЕФдЊЫиЮЛжУЃК_________
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПгаМзЁЂввСНжжЮяжЪЃК

ЃЈ1ЃЉгЩМззЊЛЏЮЊввашОЯТСаЙ§ГЬЃЈвбТдШЅИїВНЗДгІЕФЮоЙиВњЮяЃЌЯТЭЌЃЉЃК
![]()
ЦфжаЗДгІIЕФЗДгІРраЭЪЧ___________ЃЌЗДгІIIЕФЬѕМўЪЧ_______________ЃЌЗДгІIIIЕФЛЏбЇЗНГЬЪНЮЊ___________________________________ЃЈВЛашзЂУїЗДгІЬѕМўЃЉЁЃ
ЃЈ2ЃЉЯТСаЮяжЪВЛФмгыввЗДгІЕФЪЧ ЃЈбЁЬюађКХЃЉЁЃ
aЃЎН№ЪєФЦ bЃЎфхЫЎ cЃЎЬМЫсФЦШмвК dЃЎввЫс
ЃЈ3ЃЉввгаЖржжЭЌЗжвьЙЙЬхЃЌШЮаДЦфжавЛжжФмЭЌЪБТњзуЯТСаЬѕМўЕФЭЌЗжвьЙЙЬхНсЙЙМђЪН ЁЃ
aЃЎБНЛЗЩЯЕФвЛТШДњЮягаСНжж
bЃЎгіFeCl3ШмвКЯдЪОзЯЩЋ
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com