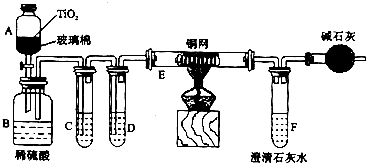
��������1����ԭ���غ�͵�ʧ�����غ���д��̿��ˮ�ڸ�������ȡ�ϳ��������ΪH
2��CO���Ļ�ѧ����ʽ��
��2������CO��CH
3OH��ȼ��������д�ȷ���ʽ�������ø�˹�����������״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽ��
��3����A����Ӧ�����Ƿ�Ӧ��ij���ʵ�Ũ���ڵ�λʱ���ڵı仯���ݴ˿��жϣ�
B�����ݷ�ӦCO
2��g��+3H
2��g��?CH
3OH��g��+H
2O��g���������ı仯�����ɴ��ж��¶ȱ仯ʱƽ���ƶ��ķ������ж�ƽ�ⳣ���ı仯��
C�������¶ȵı仯��ƽ���Ӱ�죬�ж�ƽ���ƶ��ķ����پݴ��жϣ�
D�������¶ȱ仯�������������ʵ�����Ӱ�죬���ж�ƽ���ƶ�����
��4�����ݻ�ѧƽ������η�����ƽ��ʱ�����ʵ����ʵ����������÷�Ӧǰ����������ʵ���֮�ȵ���ѹǿ֮�������
��5������ԭ����и�������������Ӧ������������ԭ��Ӧ�������ǵ������Һ����缫��Ӧ��������̼������Һ��̼�������ˮ����Һ�ʼ��ԣ�̼������ӷֲ�ˮ�⣻
����������ʱ��AgCl��Һ��c��Ag
+��=
=
mol/L��AgBr��Һ��c��Ag
+��=
=
mol/L��
Ag
2CrO
4��Һ��c��Ag
+��=
=
mol/L��c��Ag
+��ԽС�������ɳ�����
���
�⣺��1����ԭ���غ�͵�ʧ�����غ���д��̿��ˮ�ڸ�������ȡ�ϳ��������ΪH
2��CO���Ļ�ѧ����ʽΪ��C+H
2O
CO+H
2���ʴ�Ϊ��C+H
2O
CO+H
2��
��2����CO��g����CH
3OH��l����ȼ���ȡ�H�ֱ�Ϊ-283.0kJ?mol
-1��-726.5kJ?mol
-1����
��CO��g��+1/2O
2��g��=CO
2��g����H=-283.0kJ?mol
-1 ��CH
3OH��l��+3/2O
2��g��=CO
2��g��+2 H
2O��l����H=-726.5kJ?mol
-1 �ɸ�˹���ɿ�֪�â�-�ٵ÷�ӦCH
3OH��l��+O
2��g��=CO��g��+2 H
2O��l�����÷�Ӧ�ķ�Ӧ�ȡ�H=-726.5kJ?mol
-1-��-283.0kJ?mol
-1��=-443.5kJ?mol
-1��
�ʴ�Ϊ��CH
3OH��l��+O
2��g��=CO��g��+2 H
2O��l����H=-443.5kJ?mol
-1��
��3����A����Ӧ�����Ƿ�Ӧ��ij���ʵ�Ũ���ڵ�λʱ���ڵı仯��v��CH
3OH��=
��mol?L
-1?min
-1������A��ȷ��
B�����ݷ�ӦCO
2��g��+3H
2��g��?CH
3OH��g��+H
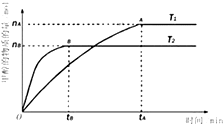
2O��g���Ƿ��ȷ�Ӧ��֪�������¶ȣ�ƽ�����淴Ӧ�����ƶ����÷�Ӧ��T
1ʱ��ƽ�ⳣ����T
2ʱ�Ĵ�B��ȷ��
T
2��T
1��T
2ƽ��ʱ���״��������٣���˵�����淴ӦCO
2+3H
2?CH
3OH+H
2O���淴Ӧ�����ƶ���������ӦΪ���ȷ�Ӧ����B��ȷ��
C�������¶ȣ�ƽ�����淴Ӧ�����ƶ�����ӦΪ���ȷ�Ӧ����C����
D������A��ķ�Ӧ��ϵ��T
1�䵽T
2�������¶ȣ��״������ʵ�����С��ƽ�����淴Ӧ�����ƶ���
����D����
�ʴ�Ϊ��AB��
��4���ɻ�ѧƽ�������ģʽ�������֪��
CO
2 ��g��+3H
2��g��=CH
3OH��g��+H
2O��g��
��ʼ 1 2 0 0
�仯 a 3a a a
ƽ�� 1-a 2-3a a a
������ͬ�����������ѹǿ֮�ȵ������ʵ���֮�ȣ�
�������ڵ�ѹǿ����ʼѹǿ֮��Ϊ=��1-a+2-3a+a+a������1+2��=��3-2a����3��
�ʴ�Ϊ����3-2a����3��
��5����ȼ�ϵ����ԭ��ص�һ�֣�����ʧ���ӷ���������Ӧ�������õ��ӷ�����ԭ��Ӧ���״�ȼ�����ɶ�����̼��ˮ��
���ڼ��Խ����У����������������Ӳ��뷴Ӧ���״�ȼ�ϵ�صĸ�����ӦʽΪCH
3OH+8OH
--6e
-=CO
32-+6H
2O��Na
2CO
3��Һ�д���ˮ��ƽ��CO
32-+H
2O?HCO
3-+OH
-��HCO
3-+H
2O?H
2CO
3+OH
-����Һ�ʼ��ԣ���Һ������Ũ�ȴ�СΪC��Na
+����C��CO
32-����C��OH
-����C��HCO
3-����C��H
+����
�ʴ�Ϊ��CH
3OH+8OH
--6e
-=CO
32-+6H
2O��C��Na
+����C��CO
32-����C��OH
-����C��HCO
3-����C��H
+����
����������ʱ��AgCl��Һ��c��Ag
+��=
=
mol/L=1.56��10
-8mol/L��
AgBr��Һ��c��Ag
+��=
=
mol/L=7.7��10
-11mol/L��
Ag
2CrO
4��Һ��c��Ag
+��=
=
mol/L=3��10
-5��c��Ag
+��ԽС����Խ�����ɳ����������������Ӳ����������Ⱥ�˳��ΪBr
-��Cl
-��CrO
42-���ʴ�Ϊ��Br
-��Cl
-��CrO
42-��

 �״���һ�����͵���Դ��
�״���һ�����͵���Դ��


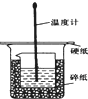 50ml0.50mol?L-1������50mL0.55mol?L-1NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���� �зų��������ɼ����к��ȣ��ش��������⣺
50ml0.50mol?L-1������50mL0.55mol?L-1NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���� �зų��������ɼ����к��ȣ��ش��������⣺