
����Ŀ��Ϊ̽��ʵ��������ϩ����ϩ����ˮ�ļӳɷ�Ӧ����ͬѧ�������ͼ��ʾ��ʵ��װ�ã���������ʵ�顣���¶�����170������ʱ���д����������������������ͨ����ˮ�У���ˮ����ɫѸ����ȥ����ͬѧ��Ϊ�ﵽ��ʵ��Ŀ�ġ���ͬѧ��ϸ�۲��˼�ͬѧ������ʵ����̣����ֵ��¶�����100������ʱ����ɫҺ�忪ʼ��ɫ����160������ʱ�����Һȫ�ʺ�ɫ����170�泬�������������ٶ����Լӿ죬���ɵ������д̼�����ζ���ɴ����Ƴ���������������Ӧ�����ʣ�����Ӱ����ϩ�ļ���������ȥ���ݴ˻ش��������⣺

��1��д����ͬѧʵ������Ϊ�ﵽʵ��Ŀ�ĵ�������Ӧ�Ļ�ѧ����ʽ:__________________��
��2����ͬѧ�۲쵽�ĺ�ɫ������__________���̼���������__________����ͬѧ��Ϊ�̼�������Ĵ��ھͲ�����Ϊ��ˮ��ɫ����ϩ�ļӳɷ�Ӧ��ɵġ�ԭ���ǣ��û�ѧ���̱�ʾ����________________________________ ��
��3����ͬѧ���ݼ���ͬѧ�ķ�������Ϊ��������CO��CO2�������������Ϊ֤��CO���ڣ�����������¹��̣��ù��̿ɰ�ʵ���в������л����������������������徭��ȼ����ɫ���棬ȷ����һ����̼��
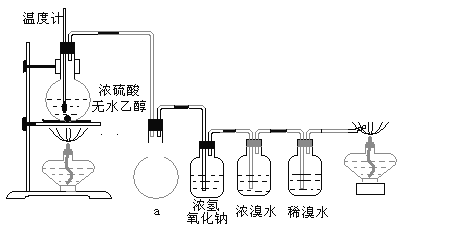
�����װ��a��������_____________________________________________
��Ũ��ˮ��������_________________________________________________��
ϡ��ˮ��������___________________________________________________��
���𰸡���11�֣��� CH3CH2OH![]() CH2��CH2����H2O ��2�֣�
CH2��CH2����H2O ��2�֣�
CH2��CH2��Br2��Br��CH2CH2��Br ��2�֣�
�� C��1�֣���SO2��1�֣���Br2��SO2��2H2O��2HBr��H2SO4��2�֣�
�Ǣ���ȫװ�ã���������1�֣�
��������ϩ�������������壨1�֣���������ϩ���������������Ƿ������1�֣�
��������
���⣨1�������Ҵ���Ũ����Ĵ������·�����������ˮ��ȡ��ϩ���Ҵ���������ȥ��Ӧ��CH3CH2OH![]() CH2==CH2��+ H2O����ˮ�е�������ϩ�����ӳɷ�Ӧ����CH2=CH2+Br2��CH2BrCH2Br��
CH2==CH2��+ H2O����ˮ�е�������ϩ�����ӳɷ�Ӧ����CH2=CH2+Br2��CH2BrCH2Br��
��2����ͬѧ��ϸ�۲��˼�ͬѧ������ʵ����̣����ֵ��¶�����100������ʱ����ɫҺ�忪ʼ��ɫ����160������ʱ�����Һȫ�ʺ�ɫ��Ũ�������ǿ�����ԣ��������Ҵ������Ҵ�������̼��ͬʱ��������ԭ�ɶ���������ˮ�����������������ԭ��Ӧ���屻����������+4�۵���ԭ��-1�۵��壬+4�۵�����������+6�۵�����ˮ��ɫ������ʽΪ��Br2+ SO2+ 2H2O ="=" 2HBr + H2SO4 ���ʴ�Ϊ��C��SO2��Br2+ SO2+ 2H2O ="=" 2HBr + H2SO4 ��
��3����ϩ����ˮ�е��巢���ӳɷ�Ӧ����ˮ��ɫ���ɼ�����ϩ�Ĵ��ڣ�����������巢��������ԭ��Ӧ��������������ᣬ�ɼ����������Ĵ��ڣ������װ��a�������ǣ���ȫװ�ã�����������Ũ��ˮ�������ǣ�������ϩ��������ϩ�Ͷ����������壩��ϡ��ˮ�������ǣ�������ϩ�Ƿ������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2019�����Ž��з����Ԫ�����ڱ�150���꣬���Ϲ���2019�궨Ϊ�����ʻ�ѧԪ�����ڱ���������֪������Ԫ��W��X��Y��Z�����ڱ��е����λ����ͼ��ʾ��������Ԫ�ص�ԭ������������֮��Ϊ24������˵����ȷ���ǣ� ��

A. W���⻯����Z���⻯�ﷴӦ�������ˮ��Һ�ʼ���
B. ��Ԫ�����ڱ��У�117��Ԫ����ZԪ��λ��ͬһ����
C. �������Ӧˮ��������ԣ�Z��Y
D. W��X��Y��Z����Ԫ�ض�Ӧ�ĵ��ʵķе㣺W��X��Y��Z
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)15.6gNa2X�����к���0.4molNa+����û������Ħ������Ϊ______������ЩNa2X�����ܽˮ�У�����ˮϡ����2L����������Һ��Na+�����ʵ���Ũ��Ϊ____ mol��L-1��
(2)��ⱥ��ʳ��ˮ�Ļ�ѧ����ʽΪ___________����ʱ����117gNaCl�������������ɵõ�_____ L(��״��)������
(3)����2mol����ͨ������ʯ�����У������Ͽɵõ��������________�ˣ�(д������������)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ���ܱ��������м���һ�����ɻ����ĸ��壨��Ȳ��ƣ��������ֳ������֣�����߳���8molN2���ұ߳���CO��CO2�Ļ�����干64gʱ�����崦����ͼλ�ã������¶Ȳ��䣩������˵����ȷ����

A. ���CO��CO2������֮��Ϊ1:3
B. �ұ�CO������Ϊ14g
C. �ұ������ܶ�����ͬ�����������ܶȵ�2��
D. ���ı��ұ�CO��CO2�ij�������ʹ���崦�ھ����Ҷ�![]() �����������¶Ȳ��䣬��ǰ�����������ڵ�ѹǿ֮��Ϊ5:6
�����������¶Ȳ��䣬��ǰ�����������ڵ�ѹǿ֮��Ϊ5:6
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ҵ��ˮ�н����±������е�5��(������ˮ�ĵ��뼰���ӵ�ˮ��)���Ҹ������ӵ����ʵ���Ũ����ȣ���Ϊ0.1 mol/L��
������ | K����Mg2����Fe3����Al3����Fe2�� |
������ | Cl���� |
ijͬѧ��̽����ˮ����ɣ�����������ʵ�飺
���ò�˿պȡ������Һ���ڻ��������գ�����ɫ����(����ɫ�ܲ����۲�)��
��ȡ������Һ������KSCN��Һ�����Ա仯��
����ȡ��Һ�����������ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ����ʱ��Һ��Ȼ���壬����Һ������������䡣
�����������õ���Һ�м���BaCl2��Һ���а�ɫ�������ɡ�
���ƶϣ�
(1)�������������жϣ���Һ��һ�������е���������______________��һ�������е���������_____________(д���ӷ���)��
(2)���м�����������������ɫ��������ӷ���ʽ��___________________________���������ɰ�ɫ���������ӷ���ʽ��___________________________��
(3)���������ú���ɫ����ͨ��ˮ�У��������ɫ����������Ӧ�Ļ�ѧ����ʽΪ__________________________��
(4)��ͬѧ����ȷ��ԭ��Һ��������������___________����������________________д���ӷ���)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��1684 �꣬�����������һ����Ϊ����Ѫ����Ȼʷ�ԡ���ҽѧ���������Ȿ��� ���ܽ����Լ���ѪҺ���������ʵ��ɹ���������ʷ�����������һ�ν���ѧ������������ �ٴ�ҽѧ��ͨ��ʵ�飬�����֤����ѪҺ�к����Ȼ��ƣ�����֮��Ϊ�̶��Ρ�������Ա�Ӻ�ˮ�õ��Ĵ����к�����ɳ�Ȳ��������ʣ��Լ����������ʣ�Ca2+��Mg2+��SO42-�ȣ��ᴿ����������ͼ��
��1���������������Ƚ������ܽ⣬��ͨ������ʵ�鲽������ᴿ���ٹ��ˢڼӹ��� NaOH �� Һ�ۼ���������ܼӹ��� Na2CO3 ��Һ�ݼӹ��� BaCl2 ��Һ��
���²���˳��������_____������ĸ��
A���ڢݢܢۢ� B���ܢݢڢ٢� C���ݢڢܢ٢� D���ݢܢڢ٢�
��ȥ Mg2+�����ӷ���ʽ_________��
��2��ʵ���ҽ������õ��ľ���ʳ��ˮ�Ƴɾ��εĹ����У�����Ҫ����ijһ�������ò����� ��Ҫ���ȵ�����Ϊ��_____��
��3������ʵ�����������þƾ���ֱ�Ӽ��ȵ���_______������ĸ��
���Թ� ����Ͳ ��Բ����ƿ ���ձ� ������ƿ ����ƿ ��ȼ�ճ�
A. �٢ۢܢޢ� B. �٢� C. �٢ۢܢݢ� D. �٢ڢۢܢݢޢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijѧ��Ϊȷ��ij���ķ���ʽ������ܵĽṹ��ʽ����������ʵ�飺��״���£�ȡ0.1 mol����������������ȫȼ�գ����ɵĶ�����̼���Ϊ22.4 L��ͬʱ�õ�16.2 gˮ��
(1)������Ħ������Ϊ________��
(2)�����ķ���ʽΪ________��
(3)������һ�ֿ��ܵĽṹΪ  ������________(ѡ�����)��
������________(ѡ�����)��
A������ B������ C�������� D����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�����Һ�пɷ������·�Ӧ��
��![]()
��![]()
��![]()
����������Ӧ�ṩ����Ϣ���ж����н��۲���ȷ����
A.�����ԣ�XO4��>B2>A3+
B.X2+���л�ԭ�ԣ�����������
C.��ԭ�ԣ�Z��>A2+
D.��Һ�пɷ�����![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������һƿ�����Ȼ��Ƶ��������ƹ����Լ������ⶨNaOH����������ԼΪ82.0%��Ϊ����֤�䴿�ȣ���Ũ��Ϊ0.2mol/L�Ĵ�����еζ����Իش��������⣺
(1)������ƽ����5.0g�����Լ������500mL����Һ���á���������װ����ͼ��ʾ25.00mL��_______�ζ����У�ѡ����������������������Һ��λ�á�

(2)ȡ20.00mL����Һ������_______��ָʾ�����ζ��յ�ʱ������Ϊ_____________��
(3)�ζ����յ������3��ʵ�����ƽ����ȥ20.00mL������NaOH����������Ϊ________��
(4)�Է����ζ���������������Щʵ����������_______��
A.���ô���Һ�����У���Һת��������ƿʱ��δϴ���ձ�
B.��ʽ�ζ���������ˮϴ�Ӻ�ֱ��װ��Һ
C.�ζ�ʱ����Ӧ����ҡ��̫���ң�����������Һ����
D.�ζ����յ�ʱ���ζ��ܼ�������Һ��
E.���ζ��ܿ�ʼʱ���ӣ����յ�ʱ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com