
����Ŀ����.NH4Al(SO4)2��ʳƷ�ӹ�����Ϊ��ݵ�ʳƷ���Ӽ������ڱ���ʳƷ��NH4HSO4�ڷ����Լ���ҽҩ�����ӹ�ҵ����;�㷺����ش��������⣺
(1)��ͬ�����£�0.1 mol��L��1NH4Al(SO4)2��c(NH4+)________(������������������������С����)0.1 mol��L��1NH4HSO4��c(NH4+)��
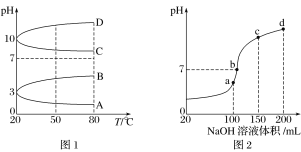
(2)��ͼ1��0.1 mol��L��1�������Һ��pH���¶ȱ仯��ͼ��
�����з���0.1 mol��L��1NH4Al(SO4)2��pH���¶ȱ仯��������________(����ĸ)��
������ʱ��0.1 mol��L��1NH4Al(SO4)2��2c(SO42-)��c(NH4+)��3c(Al3��)��________mol��L��1(����ֵ����ʽ)��
(3)����ʱ����100 mL 0.1 mol��L��1NH4HSO4��Һ�еμ�0.1 mol��L��1NaOH��Һ���õ�����ҺpH��NaOH��Һ����Ĺ�ϵ������ͼ2��ʾ���Է���ͼ��a��b��c��d�ĸ��㣬ˮ�ĵ���̶�������____________����b�㣬��Һ�и�����Ũ���ɴ�С������˳����______________��
��.pC��ָ��ϡ��Һ���������ʵ���Ũ�ȵij��ö�����ֵ������pH����ij��Һ���ʵ�Ũ��Ϊ1��10��3mol��L��1�������Һ�и����ʵ�pC����lg10��3��3����֪H2CO3��Һ�д�������ƽ�⣺CO2��H2O![]() H2CO3��H2CO3
H2CO3��H2CO3![]() H����HCO3-��HCO3-
H����HCO3-��HCO3-![]() H����CO32-ͼ3ΪH2CO3��HCO3-��CO32-�ڼ���ǿ���ǿ����Һ�ﵽƽ��ʱ��Һ�����ֳɷֵ�pCpHͼ����ش��������⣺
H����CO32-ͼ3ΪH2CO3��HCO3-��CO32-�ڼ���ǿ���ǿ����Һ�ﵽƽ��ʱ��Һ�����ֳɷֵ�pCpHͼ����ش��������⣺

(1)��pH��9ʱ��H2CO3��Һ��Ũ�����ĺ�̼Ԫ�ص�����Ϊ______��
(2)pH<4ʱ����Һ��H2CO3��pC����Լ����3��ԭ����____________________��
���𰸡�С�� A 10��3��10��11 a c(Na��)>c(SO42-)>c(NH4+)>c(OH��)��c(H��) HCO3- c(H��)�����H2CO3![]() H����HCO3-ƽ�������ƶ����ų�CO2��̼��Ũ�ȱ��ֲ���
H����HCO3-ƽ�������ƶ����ų�CO2��̼��Ũ�ȱ��ֲ���
��������
��.(1) NH4Al(SO4)2��Al3+ˮ�����������NH4+ˮ�⣬HSO4-�����H+ͬ������NH4+ˮ�⣻
(2)��NH4Al(SO4)2ˮ�⣬��Һ�����ԣ������¶���ˮ��̶�����
�ڸ��ݵ���غ㶨�ɽ��⣻
(3) a��b��c��d�ĸ��㣬���ݷ�Ӧ���Ĺ�ϵ��a��ǡ��������H+����Һ��ֻ��(NH4)2SO4��Na2SO4��b��c��d������Һ������NH3H2O��(NH4)2SO4���Դٽ�ˮ�ĵ��룬��NH3H2O����ˮ�ĵ��룮b����Һ�����ԣ�
��.(1) ����pC����֪��pCֵԽ������Ũ��ԽС����֮��pCֵԽС������Ũ��Խ����ͼ������жϣ�
(2) ������Ũ��Խ��Խ����̼����룻�����ж�����̼���ɡ�
��.(1) NH4Al(SO4)2��NH4HSO4�е�NH4+������ˮ�⣬����NH4Al(SO4)2��Al3+ˮ�����������NH4+ˮ�⣬HSO4-�����H+ͬ������NH4+ˮ�⣬��ΪHSO4-�������ɵ�H+Ũ�ȱ�Al3+ˮ�����ɵ�H+Ũ�ȴ�����NH4HSO4��NH4+ˮ��̶ȱ�NH4Al(SO4)2�е�С��
(2)��NH4Al(SO4)2ˮ�⣬��Һ�����ԣ������¶���ˮ��̶�����pH��С�����ϵ�����ΪA��
�ڸ��ݵ���غ㣬�������2c(SO42-)-c(NH4+)-3c(Al3+)=c(H+)-c(OH-)=(10��3��10��11)molL-1��
(3) a��b��c��d�ĸ��㣬���ݷ�Ӧ���Ĺ�ϵ��a��ǡ��������H+����Һ��ֻ��(NH4)2SO4��Na2SO4��b��c��d������Һ������NH3H2O��(NH4)2SO4���Դٽ�ˮ�ĵ��룬��NH3H2O����ˮ�ĵ��룮b����Һ�����ԣ�����Һ����(NH4)2SO4��Na2SO4��NH3H2O���ֳɷ֣�a��ʱc(Na+)=c(SO42-)��b��ʱc(Na+)��c(SO42-)������NԪ����SԪ�صĹ�ϵ�����Եó�c(SO42-)��c(NH4+)����c(Na+)��c(SO42-)��c(NH4+)��c(OH-)=c(H+)��
��.(1) ����pC����֪��pCֵԽ������Ũ��ԽС����֮��pCֵԽС������Ũ��Խ����ͼ��֪��pH=9ʱ��pC��С����HCO3-������HCO3-Ũ�����
(2) pH��4ʱ����Һ�У�c(H+)�����H2CO3H++HCO3-ƽ�������ƶ��ų�CO2������̼��Ũ�Ȳ��䣬̼��Ϊ������Һ��������Һ��H2CO3��pC����Լ����3��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ�����Ʊ���ˮ����ͭ[Cu(HCOO)24H2O]����ʵ�鲽�����¡�
(1)��ʽ̼��ͭ���Ʊ���
a.����i�ǽ�һ����������̼�����ƹ���һ��ŵ��в�����ĥ����Ŀ����______��
b.����ii���ڽ����½���������ֶ�λ���������ˮ�У���Ӧ�¶ȿ�����70�桪80�棬�������_____(дʵ������)��˵���¶ȹ��ߡ�
c.��صĻ�ѧ����ʽ______��
(2)��ˮ����ͭ������Ʊ�������ʽ̼��ͭ��������ձ��У�����һ�����ȵ�����ˮ������μ����������ʽ̼��ͭǡ��ȫ���ܽ⣬���ȹ��˳�ȥ�������������ʣ�Ȼ��������ȴ���ˣ�����������ˮ�Ҵ�ϴ�Ӿ���2��3�����ɣ��õ���Ʒ��
a.��صĻ�ѧ����ʽ______��
b.���ȹ����У�������ȵ�ԭ����______��
c.���Ҵ�ϴ�Ӿ����Ŀ��______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ƿ��ʢ������X���ι���ʢ��Һ��Y������ѹ��ͷ�ιܣ�ʹҺ��Y����ƿ�У�����һ��ɼ�С����a��������X��Һ��Y�������ǣ� ��

A.X��NH3��Y��ˮ
B.X��SO2��Y��NaOHŨ��Һ
C.X��CO2��Y��ϡ����
D.X��HCl��Y��NaNO3ϡ��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25��ʱ��10 mL 0.1mol��L��1Na2CO3��Һ����μ���0.1 mol��L��1HCl��Һ20 mL����Һ�в��ֺ�̼�������ʵ�������ҺpH�ı仯��ͼ��ʾ������˵������ȷ����

A��HCl��Һ�μ�һ��ʱ����ҺpH>7
B������Һ��pH=8ʱ����Һ��NaHCO3�����ʵ���Ũ��Ϊ0.1mol��L��1
C��0.1 mol��L��1Na2CO3��Һ��c(Na+)+c(H+)=c(OH��)+2c(CO32��)+c(HCO3��)
D����M����c(Na+)>c(CO32��)=c(HCO3��)>c(OH��)>c(H+)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼ��ʾװ�ý�������ʵ��,�ܵó���Ӧʵ����۵���(����)
ѡ�� | �� | �� | �� | ʵ����� |
|
A | Ũ���� | MnO2 | NaBr��Һ | ������Cl2>Br2 | |
B | Ũ��ˮ | ��ʯ�� | AgNO3��Һ | AgOH�������� | |
C | Ũ���� | Na2SO3 | FeCl3��Һ | SO2���л�ԭ�� | |
D | ϡ���� | Na2CO3 | Na2SiO3��Һ | �ǽ����ԣ�Cl>C>Si |
A.AB.BC.CD.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ⱥ����ܱ�������ͨ��SO2��NO2��һ��������ʹ��ӦSO2��g��+NO2��g��SO3��g��+NO��g���ﵽƽ�⣬����Ӧ������ʱ��仯��ʾ��ͼ������ʾ����ͼ�ɵó�����ȷ�����ǣ� ��
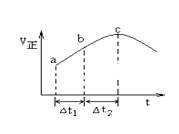
A.��Ӧ��c��ﵽƽ��״̬
B.��Ӧ��Ũ�ȣ�a��С��b��
C.��Ӧ��������������������������
D.��t1=��t2ʱ��SO2��ת���ʣ�a��b��С��b��c��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������Ƽ�ȩ(xNaHSO2��yHCHO��zH2O)�׳Ƶ��飬��ӡȾ��ҽҩ�Լ�ԭ���ܹ�ҵ���й㷺Ӧ�á�������ɿ�ͨ������ʵ��ⶨ��
��ȷ��ȡ1.540 0 g��Ʒ����ȫ����ˮ���100 mL��Һ��
�� ȡ25.00 mL������Һ��AHMT�ֹ��ȷ���ü�ȩ���ʵ���Ũ��Ϊ0.10 mol��L��1��
�� ��ȡ25.00 mL������Һ�������������ȫ��Ӧ����BaCl2��Һ��������ȫ�����ˡ�ϴ�ӡ����������صõ���ɫ����0.5825g��
���������Ƽ�ȩ�͵ⷴӦ�ķ���ʽ���£�xNaHSO2��yHCHO��zH2O��I2�D��NaHSO4��HI��HCHO��H2O(δ��ƽ)
��1������0.582 5 g��ɫ����ʱ����Ҫ���ĵ������Ϊ________��
��2�����������Һ�м����������ƣ���ȩ�ᷢ������������ԭ��Ӧ���������ֺ����л��д���÷�Ӧ�����ӷ���ʽ________________________________��
��3��ͨ������ȷ�����������Ƽ�ȩ�����(д���������)��__________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����û�ѧ��Ӧԭ���о��������ȵ��ʼ��仯����ķ�Ӧ����Ҫ���塣
��1���ϳɰ���Ӧ��ӦN2(g)+3H2(g)![]() 2NH3(g)�����ں��¡���ѹ��������ƽ����ϵ��ͨ�������ƽ��_______�ƶ�����������������������������������ʹ�ô���_____��Ӧ����H����������������С���������ı�������
2NH3(g)�����ں��¡���ѹ��������ƽ����ϵ��ͨ�������ƽ��_______�ƶ�����������������������������������ʹ�ô���_____��Ӧ����H����������������С���������ı�������
��2��O2(g)= O+2(g)+e- ![]() H1=1175.7kJ��mol-1
H1=1175.7kJ��mol-1
PtF6(g)+ e-1![]() PtF6-(g)
PtF6-(g)![]() H2= -771.1 kJ��mol-1
H2= -771.1 kJ��mol-1
O2+PtF6-(s)=O2+(g)+PtF6- ![]() H3=482.2 kJ��mol-1
H3=482.2 kJ��mol-1
��ӦO2��g��+ PtF6 (g) = O2+PtF6- (s)��![]() H=_____________ kJ��mol-1��
H=_____________ kJ��mol-1��
��3����25���£���Ũ�Ⱦ�Ϊ0.1 mol��L-1��MgCl2��CuCl2�����Һ����μ��백ˮ��������__________�������ѧʽ�������ɸó��������ӷ���ʽΪ____________����֪25��ʱKsp[Mg(OH)2]=1.8��10-11,KsP[Cu(OH)2]=2.2��10-20��
��4����25���£���a mol��L-1�İ�ˮ��0.01 mol��L-1������������ϣ���Ӧƽ��ʱ��Һ��c(NH4+)=c(Cl-)������Һ��_____________�ԣ����������������������������ú�a�Ĵ���ʽ��ʾNH3��H2O�ĵ��볣��Kb=__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���й���ѧ���о���һ�������Ϲ����[̼����(CQDs)/����̼(C3N4)��������]��������̫����ʵ�ָ�Ч�ֽ�ˮ����ԭ����ͼ��ʾ������˵����ȷ����
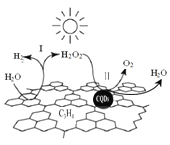
A. C3N4��C�Ļ��ϼ�Ϊ -4
B. ��Ӧ�������ξ�Ϊ���ȹ���
C. ���У�H2O2���������������ǻ�ԭ��
D. ͨ���÷�Ӧ��ʵ���˻�ѧ����̫���ܵ�ת��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com