
| 1 |
| 8 |
| 1 |
| 2 |
| 1 |
| 8 |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������� |
| B��������������̿���� |
| C������������ˮ |
| D��HCl��������ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ú������ | B��ʯ�͵ķ��� |
| C��ʯ�͵��ѽ� | D��ú�ĸ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ij�ݻ�������ܱ������У����淴ӦA��g��+B��g��?xC��g������������ͼ����ʾ��ϵ���ɴ��ƶ϶�ͼII����ȷ˵���ǣ�������
��ij�ݻ�������ܱ������У����淴ӦA��g��+B��g��?xC��g������������ͼ����ʾ��ϵ���ɴ��ƶ϶�ͼII����ȷ˵���ǣ�������| A����p3��p4��Y��ɱ�ʾB���������� |
| B����p3��p4��Y��ɱ�ʾA��ת���� |
| C����p3��p4��Y��ɱ�ʾ��������ƽ��Ħ������ |
| D����p3��p4��Y��ɱ�ʾ���������ܶ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ԫ�ش��� | A | B | C | D | E |
| ԭ�Ӱ뾶/nm | 0.160 | 0.143 | 0.102 | 0.099 | 0.074 |
| ��Ҫ���ϼ� | +2 | +3 | +6��-2 | -1 | -2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��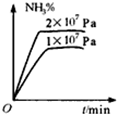 |
B��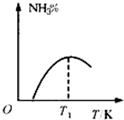 |
C��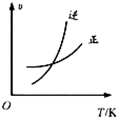 |
D��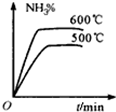 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com