
����Ŀ��ǰ36��Ԫ��A��B��C��D��Eԭ��������������A��B��ͬһ���ڽ���Ԫ�أ�B��Dͬһ���壬BԪ���⻯���ˮ��Һ�����ڲ����ĵ�̡�CԪ����ͬ����Ԫ���е�һ��������С��Ԫ�أ�C��E��������������ͬ��EԪ���ڲ���������ӡ�
��1����̬ԭ��E�ĵ����Ų�ʽΪ___________�����ݵ����Ų����ڱ�����Ϊ5��������Ԫ��λ�����ڱ���_________����
��2��Ԫ��A��B��D�縺���ɴ�С��˳��Ϊ___________��
��3��D2A�����У�����ԭ�ӵ��ӻ���ʽΪ___________�����Ӽ��Ǵ�С��ϵ��AB2����_____D2A����(����ڡ�����С�ڡ����ڡ�)��ԭ��_________________________��
��4������C�������ڴ��⣬������γɾ���ṹ��ͼ��ʾ��
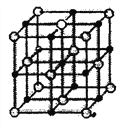
�þ�������C���ӽ��ڵ���������_______������Щ�����ӣ����ɵĿռ乹��Ϊ_______����֪C������������֮������ĺ˼��Ϊa pm����þ�����ܶ�Ϊ_____g��cm-3��
���𰸡� [Ar]3d104s1 ds F��O��Cl sp3 С�� F�ĵ縺�Դ���Cl��F��O�γɵĹ��õ��ӶԸ�ƫ����Oԭ�ӣ��γɹ��ۼ��ĵ��Ӷ��ų���������С 6 ������ ![]()
��������ǰ36��Ԫ��A��B��C��D��Eԭ��������������BԪ���⻯���ˮ��Һ�����ڲ����ĵ�̣�BΪFԪ�أ�A��B��ͬһ���ڽ���Ԫ�أ���AΪOԪ�أ�B��Dͬһ���壬��DΪCl��BrԪ�أ�CԪ����ͬ����Ԫ���е�һ��������С��Ԫ�أ���CΪNaԪ�أ�C��E��������������ͬ��EԪ���ڲ���������ӣ�E�ļ۵����Ų�ʽΪ3d104s1��EΪCuԪ�أ���DΪClԪ�ء�
(1)��̬Cuԭ�ӵĵ����Ų�ʽΪ[Ar]3d104s1�����ݵ����Ų������ڱ�����Ϊ5��������Ԫ��λ�����ڱ���ds�����ʴ�Ϊ��[Ar]3d104s1��ds��
(2)Ԫ�صķǽ�����Խǿ���縺����ֵԽ��Ԫ��O��F��Cl�縺���ɴ�С��˳��ΪF��O��Cl���ʴ�Ϊ��F��O��Cl��
(3)Cl 2O�����У�����ԭ��Oԭ�ӵļ۲���Ӷ���=2+![]() (6-2��1)=4������sp3�ӻ���F�ĵ縺�Դ���Cl��F��O�γɵĹ��õ��ӶԸ�ƫ����Oԭ�ӣ��γɹ��ۼ��ĵ��Ӷ��ų���������С��ʹ�÷����еļ���OF2���ӣ�Cl 2O���ӣ��ʴ�Ϊ��sp3��С�ڣ�F�ĵ縺�Դ���Cl��F��O�γɵĹ��õ��ӶԸ�ƫ����Oԭ�ӣ��γɹ��ۼ��ĵ��Ӷ��ų���������С��
(6-2��1)=4������sp3�ӻ���F�ĵ縺�Դ���Cl��F��O�γɵĹ��õ��ӶԸ�ƫ����Oԭ�ӣ��γɹ��ۼ��ĵ��Ӷ��ų���������С��ʹ�÷����еļ���OF2���ӣ�Cl 2O���ӣ��ʴ�Ϊ��sp3��С�ڣ�F�ĵ縺�Դ���Cl��F��O�γɵĹ��õ��ӶԸ�ƫ����Oԭ�ӣ��γɹ��ۼ��ĵ��Ӷ��ų���������С��
(4)����ͼʾ���þ������������ӽ��ڵ���������6������Щ�����ӣ����ɵĿռ乹��Ϊ�����壻�����к��е������Ӻ������Ӹ����ֱ�Ϊ8��![]() +6��
+6��![]() =4��12��
=4��12��![]() +1=4����ѧʽΪNaH����������������֮������ĺ˼��Ϊa pm����þ�����ܶ�Ϊ
+1=4����ѧʽΪNaH����������������֮������ĺ˼��Ϊa pm����þ�����ܶ�Ϊ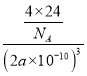 g��cm-3=
g��cm-3=![]() g��cm-3���ʴ�Ϊ��6�������壻
g��cm-3���ʴ�Ϊ��6�������壻 ![]() ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����̺Ͻ����������������ij����С�����ⶨ���̺Ͻ����̵ĺ�����
I���������[(NH4)2Fe(SO4)2]��Һ��Ũ�ȵζ�
����1:ȡ20.00mL 0.015mol/L K2Cr2O7��Һ��250mL��ƿ��,����20mLϡ�����5mLŨ����,�������������Һ�ζ�,�ӽ��յ�ʱ��2��R��Һ��ָʾ��,�����ζ����յ�,���ĵ����ΪV1mL��
����2���ظ�����1ʵ��,�ӽ��յ�ʱ��4��R��Һ�����ĵ����ΪV2mL.
���������[(NH4)2S2O8]�������ζ����ⶨ�̺���
ȡmg���̺Ͻ�����ƿ��,����������Ũ����,��������ȫ�ܽ�,ϡ����ȴҥ��,�ټ�����������������Һ�������Ĺ��������Һ,�����������������ð��,��ȴ������(��ʱ��Һ���̵Ļ��ϼ�Ϊ+7),�ñ궨�������������Һ���еζ�������ʵ�����ݼ������̺Ͻ����̵ĺ�����
[��������]������������,Cr2O72-���к�ǿ�������ԣ��ױ���ԭΪ��ɫ��Cr3+��
��R��Һ�ı�ɫԭ��: ![]()
��1�����в���1.2��Ҫ�IJ�����������ƿ����ͷ�ιܣ�_______��________��
��2�����еζ�����,��Ҫ��Ӧ�����ӷ���ʽ��________��
��3�����в���2����Ҫ������_________
��4�����������������Һ�ĵζ�Ũ����_________mol/L (�ú�V1��V2�Ĵ���ʽ��ʾ)��
��5�����м�����������Һ��Ŀ����________��
��6��������δ���������������ð�����̺����ⶨ�����_______(����ƫ������ƫС��������Ӱ����)��
��7��Ϊ����߸�ʵ���ȷ�ȺͿɿ���,�����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ�����ӵ�����ֵ�������й�������ȷ����
A. 22g N2O��CO2��ɵĻ����������������ԭ������Ϊ1��5NA
B. 0��1mol��L-1�İ�ˮ��Һ�У�NH3��H2O��NH4+����Ŀ֮��Ϊ0.1NA
C. һ��������Fe����ϡ�����У�������22.4L NOʱ��ת�Ƶ�����Ϊ3NA
D. ������78g��������Ϊ26������Ȳ�ı���Һ�У�����̼ԭ�ӵ���ĿΪ6NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���п�Ժ��������������ϸ������ij����ַ�������ͼ��ʾ��


��1��������ͼ��Ϣ���Կ��������е�������Ⱦ�ﲢ���ɻ�������ʻ��ɵ���______����������������ϡȼ����ϵͳ��Ҫ����ԭ��������ͼ��ʾ��д��ϡȼ������NO��������Ҫ��Ӧ�ķ���ʽ_____________________________________��
��2��ũҵ��ų��İ�������ʩ�õĻ��ʷֽ⣬Ҳ������ʩ�ò������µġ�����ijЩ��������Է��ϻ��ʩ�û��ͳ����������ӷ���ʽ����________________________��
��3�������о������ҹ����������ԣ�����Ҫԭ������ͼ��ʾ��A�Ļ�ѧʽ��________��
2NH3(��)+SO2(��)+2NO2(��)![]() 2NH4+(��Һ)+A(��Һ)+2HONO(��)
2NH4+(��Һ)+A(��Һ)+2HONO(��)
��4��úȼ���ŷŵ���������SO2��NOx������NaClO2��Һ��Ϊ���ռ���ͬʱ��������������������������SO2��NOx������ͨ��ʢ��NaClO2��Һ�ķ�Ӧ���У���Ӧһ��ʱ������Һ������Ũ�ȵ��й��������£������������Ӻ��Բ��ƣ���
���� | Na+ | SO42�� | NO���� | H+ | Cl |
Ũ��/��mol��L1�� | 5.5��103 | 8.5��104 | y | 2.8��104 | 3.5��103 |
��NO��NaClO2��Һ��Ӧ�����ӷ���ʽ��___________________��
�ڱ���y��_______��
��5����ҵ��������Ҳ�п��ܲ���NOx��Ⱦ����д�����������еĵ�һ�������Ĵ������Ļ�ѧ����ʽ___________________________________�����õ����ư��IJ���Ϊ90%���ð���������ʱ���Ĵ������͵�������ת��Ϊ������������Ϊ5%��3%��1000 mol��������___________mol���ᡣ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����ͼ�����߿���ÿһ�С�ÿһ���൱�ڿα���¼��Ԫ�����ڱ���ÿһ���ÿһ���ڣ����ѱ����Ԫ�ص�λ�ã�����������������������Ԫ�����ڱ����������߿�����ʵ�������ڱ���һ���������ڵ�������������������ǽ����ķֽ���________��

��2�����ֶ���������Ԫ�ص�ԭ�Ӱ뾶����Ҫ���ϼ�
Ԫ�� | �� | �� | �� | �� | �� | �� | �� | �� |
ԭ�Ӱ뾶(nm) | 0.077 | 0.143 | 0.111 | 0.104 | 0.066 | 0.186 | 0.037 | 0.099 |
��Ҫ���ϼ� | +4��-4 | +3 | +2 | +6��-2 | -2 | +1 | +1 | +7��-1 |
����Ԫ�ص�ԭ�ӽṹʾ��ͼ____________������Ԫ�����ڱ��е�λ��___________���졢��������ԭ�Ӹ�����1��1��1�γɵĻ�����Ľṹʽ______________��
�ڼĵ����붡������������ˮ���ﷴӦ�Ļ�ѧ����ʽ��___________�����ĵ����뼺������������ˮ���ﷴӦ�����ӷ���ʽ��______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ϩ�Ʒӣ��ṹ��ʽ����ͼ��ʾ������һ�ּ�����ҩ�������������ȷ����
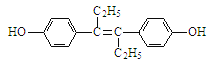
A. ��ϩ�Ʒӵķ���ʽΪC18H20O2
B. ��ϩ�Ʒ���NaOH��Һ��NaHCO3��Һ���ܷ�Ӧ
C. 1 mol��ϩ�Ʒ��������2 mol Na������Ӧ
D. ��ϩ�Ʒ��뱥����ˮ���Է����ӳɷ�Ӧ��ȡ����Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ�dz����²��ֶ�����Ԫ�أ�����������Ӧˮ����ĵ����ʵ���Ũ��ϡ��Һ��pHֵ��ԭ�������Ĺ�ϵͼ������H�����������������������˵����ȷ����

A. Ԫ��B��Ӧ���⻯���J��Ӧ���⻯���۷е���ߣ�ԭ����B���⻯���еļ��ܱ�J�еĴ�
B. ����ͼ�η�����֪��K��L����Ԫ������������Ӧ��ˮ��������ԣ�ǰ�߽�ǿ��
C. ����IC2�ۻ�ʱ�˷��Ļ�ѧ���;���KC2��ˮ��Ӧʱ�˷��Ļ�ѧ����������ͬ��
D. Ԫ��K��H��G�ֱ��γɵļ����ӵİ뾶����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������M��һ�־���������ζ����ɫ��״Һ�壬�ṹ��ʽ��![]() ����ϳ�·�����£����ַ�Ӧ����δע������
����ϳ�·�����£����ַ�Ӧ����δע������
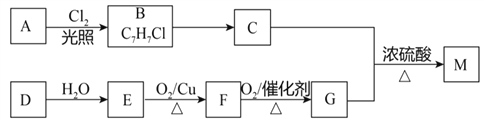
��1��B���������ŵ�������___________________��
��2��B��C��Ӧ���Լ���������___________________________��
��3����D�Ľṹ��ʽ��________________��
��4��E��F��Ӧ�Ļ�ѧ����ʽ��________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ˮ����ֳЭ����������һ�����õ绯ѧԭ�����������ˮ�ʵķ�������װ����ͼ��ʾ��
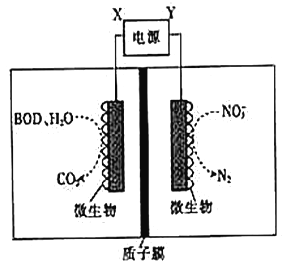
����˵���������
A. XΪ��Դ����
B. ����װ���ڸ����½��У���Ч�ʽ�����
C. ��BODΪ������(C6H12O6),��1mol�����DZ���ȫ����ʱ�������ϵ缫������24mole-
D. ����1molNO3-����ԭ������6molH+ͨ������ĤǨ����������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com