
����Ŀ����NAΪ�����ӵ�����ֵ�������й�������ȷ����
A. 22g N2O��CO2��ɵĻ����������������ԭ������Ϊ1��5NA
B. 0��1mol��L-1�İ�ˮ��Һ�У�NH3��H2O��NH4+����Ŀ֮��Ϊ0.1NA
C. һ��������Fe����ϡ�����У�������22.4L NOʱ��ת�Ƶ�����Ϊ3NA
D. ������78g��������Ϊ26������Ȳ�ı���Һ�У�����̼ԭ�ӵ���ĿΪ6NA
���𰸡�D
��������A��N2O��CO2��Ħ��������Ϊ44g/mol����22g���������ʵ���Ϊ0.5mol�������߾�Ϊ��ԭ�ӷ��ӣ���0.5mol������к�1.5NA��ԭ�ӣ���������ԭ��������A����B. δע����Һ�������������0��1mol��L-1�İ�ˮ��Һ��NH3��H2O��NH4+����Ŀ֮�ͣ���B����C. Ϊע���Ƿ�Ϊ��״������ȷ��22.4L NO�����ʵ�������C����D. ��Ȳ�ͱ������ʽ����CH��������78g��Ȳ�ı���Һ�к���CH�����ʵ���Ϊ![]() =6mol������̼ԭ�ӵ���ĿΪ6NA����D��ȷ����ѡD��
=6mol������̼ԭ�ӵ���ĿΪ6NA����D��ȷ����ѡD��


 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��̼���������CH3OCOOCH3�����DMC������һ��Ӧ��ǰ���㷺���²��ϣ�ʵ�����п��ü״���CO��CO2�Ƚ��кϳɡ��ش��������⣺
��1��������̼�ĵ���ʽΪ _________________
��2���ü״���CO��O2�ڳ�ѹ��70��120��ʹ����������ºϳ�DMC��
��֪����CO�ı�ȼ����Ϊ��283.0kJmol1��
��1mol H2O��l����ȫ�������H2O��g��������44kJ������
��2CH3OH��g����CO2��g��CH3OCOOCH3��g����H2O��g����H��15.5kJmol1
��2CH3OH��g����CO��g����1/2O2��g��CH3OCOOCH3 ��g����H2O��l����H��_____��
�÷�Ӧ�ڳ�ѹ��70��120�������¾����Է���Ӧ��ԭ����_______________��
��3����������Ӧ��֪�״���CO2��ֱ�Ӻϳ�DMC���״�ת����ͨ�����ᳬ��1%����Լ�÷�Ӧ����ҵ��������
��д���÷�Ӧƽ�ⳣ������ʽ��______________ ��
���ں����ܱ������з���������Ӧ����˵���Ѵﵽƽ��״̬����____��ѡ���ţ���
A��v����CH3OH����2v����CO2��
B��CH3OCOOCH3��H2O�����ʵ���֮�ȱ��ֲ���
C��������������ܶȲ���D��������ѹǿ����
��ij�о�С����ij�¶��£���100mL�����ܱ�������Ͷ��2.5mol CH3OH��g��������CO2��6��105 mol�������о���Ӧʱ��Լ״�ת������TON����Ӱ�죬��仯������ͼ1��ʾ�������㹫ʽΪ��TON��ת���ļ״������ʵ���/���������ʵ�������

�ڸ��¶��£���ѷ�Ӧʱ����_________h��4��10h��DMC��ƽ����Ӧ������_______ ��
��4���Զ�ײ�Ϊ�缫��������ͼװ����A��B�ڷֱ�ͨ��CH3OCOOCH3��O2����ȼ�ϵ�أ���д���õ�ظ����ĵ缫��Ӧʽ____________________________

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±�ΪԪ�����ڱ���ͬ�ڵ�һ���֣� A��B��C��D��E����Ԫ�������ڱ��е�λ������ͼ��ʾ��CԪ�ص�ԭ������������Ϊ������3����
A | E | C | |
B | D |
�ش��������⣺
��1��BԪ�������ڱ��е�λ��Ϊ__________��
��2��D������������Ӧˮ����Ļ�ѧʽΪ__________��
��3��������ʵ��˵��CԪ�صķǽ����Ա���Ԫ�صķǽ�����ǿ����__________��
a��C������H2S��Һ��Ӧ����Һ�����
b����������ԭ��Ӧ�У�1molC���ʱ�1molS�õ��Ӷ�
c��C��S��Ԫ�صļ��⻯�����ȷֽ⣬ǰ�ߵķֽ��¶ȸ�
d. CԪ�صļ��⻯��е����SԪ�صļ��⻯��
��4��B��D��Ԫ�صĵ��ʷ�Ӧ���ɻ�����M�����Ľṹ���Ƽ��飬д��M�ĵ���ʽ_____��
��5��A��þ�γɵ�1mol������N��ˮ��Ӧ������2molMg(OH)2��1mol��̬��������������̼��������Ϊ9:1��д��N��ˮ��Ӧ�Ļ�ѧ����ʽ______________________________��
��6��ͭ��һ��Ũ�ȵ����������Ļ���ᷴӦ�����ɵ���ֻ������ͭ��ͬʱ���ɵ�����������ɱ�������Ԫ����ɣ��������Է���������С��50��Ϊ��ֹ��Ⱦ����������������ȫת��Ϊ��ۺ������Σ�����1L2.2mol/LNaOH��Һ��1molO2������������ķ���ʽ�����ʵ����ֱ�Ϊ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijԪ��ԭ�ӵ�����������Ϊ������������3�������Ԫ��ԭ�Ӻ���������Ϊ(����)
A.3B.8
C.10D.7
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й���ͼʾ�ĸ�װ�õ���������ȷ����


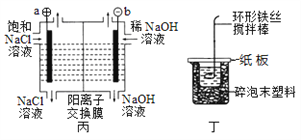
A. װ�ü��ǿ����������϶�ͭ��װ��
B. װ������Ϊ��⾫��ͭװ�ã���X�缫Ϊ��ͭ
C. װ�ñ���a�˲�����������ʹʪ��ĵ��۵⻯����ֽ����
D. װ�ö������ڲⶨ��ѧ��Ӧ�ķ�Ӧ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ǽ��㶹����һ�����Ƶ���ʯ��ҩ���ṹ��ʽ��ͼ��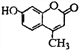 ����˵������ȷ����
����˵������ȷ����
A. �Ǽ��㶹�ط���ʽΪC10H8O3
B. 1mol�Ǽ��㶹����������5mol H2�����ӳ�
C. 1mol�Ǽ��㶹����������NaOH��Һ����������3mol NaOH
D. �Ǽ��㶹�ط����к������������ǻ���̼̼˫�������ڷ����廯����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ϩ��ʯ����ҵ����Ҫ����ԭ�ϡ��ܷ�������һϵ�б仯������˵������ȷ����
![]()
A. �л���I�ܷ���������Ӧ��ȡ����Ӧ�ͼӳɷ�Ӧ
B. �л���II��ʹ������Ȼ�̼��Һ��ɫ
C. �л�����ͬ���칹���У�������ֻ��һ�����������ʲ�ֻ4��
D. ��ͼ��Ӧ������ȡ����Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ǰ36��Ԫ��A��B��C��D��Eԭ��������������A��B��ͬһ���ڽ���Ԫ�أ�B��Dͬһ���壬BԪ���⻯���ˮ��Һ�����ڲ����ĵ�̡�CԪ����ͬ����Ԫ���е�һ��������С��Ԫ�أ�C��E��������������ͬ��EԪ���ڲ���������ӡ�
��1����̬ԭ��E�ĵ����Ų�ʽΪ___________�����ݵ����Ų����ڱ�����Ϊ5��������Ԫ��λ�����ڱ���_________����
��2��Ԫ��A��B��D�縺���ɴ�С��˳��Ϊ___________��
��3��D2A�����У�����ԭ�ӵ��ӻ���ʽΪ___________�����Ӽ��Ǵ�С��ϵ��AB2����_____D2A����(����ڡ�����С�ڡ����ڡ�)��ԭ��_________________________��
��4������C�������ڴ��⣬������γɾ���ṹ��ͼ��ʾ��
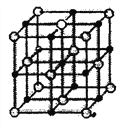
�þ�������C���ӽ��ڵ���������_______������Щ�����ӣ����ɵĿռ乹��Ϊ_______����֪C������������֮������ĺ˼��Ϊa pm����þ�����ܶ�Ϊ_____g��cm-3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��1��3-����ϩ������������ ������Ҫ�Ļ���ԭ�ϣ������ںϳɿɽ����PBS���Ϻ��¿��ᡣ
������Ҫ�Ļ���ԭ�ϣ������ںϳɿɽ����PBS���Ϻ��¿��ᡣ

��֪��
��1����Ӧ�ٵķ�Ӧ������_______________________��
��2��C�ķ���ʽ��C4H4O4����һ�ֶ�Ԫ���ᡣCΪ˳ʽ�ṹ���ṹ��ʽ��____________��
��3����Ӧ�ڵĻ�ѧ����ʽ��__________________________________________________��
��4��E�ķ���ʽC6H8O4��
i��G��������________________��
ii��H�Ľṹ��ʽ��____________________________��
��5�����¿��������ͬ�������Ŀ�Ĺ����ŵ�ͬ���칹�����Ŀ��_________��������˳���칹����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com