
����Ŀ����һ��NaCl��Na2CO3��10H2O��NaHCO3�Ļ���ijͬѧ�������ʵ�飬ͨ��������Ӧǰ��C��Dװ�������ı仯���ⶨ�û�����и���ֵ�����������
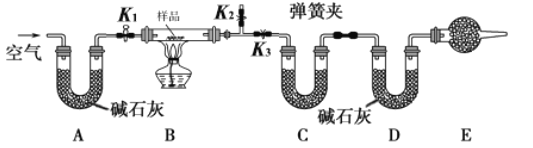
��1���뽫ʵ�鲽�貹������
�ٰ�ͼ���г�����δ��������װ��ʵ��װ�ú����Ƚ��еIJ����� _____________________��
�ڳ�ȡ��Ʒ�����������Ӳ�ʲ������У�������C��Dװ�õ�������
�۴���K1��K2���ر�K3������������������ӣ���Ŀ����____________________��
�ܹرջ���K1��K2����K3����ȼ�ƾ��Ƽ��������ٲ������塣
�ݴ���K1������������������ӣ�Ȼ��ж��װ�ã��ٴγ���C��Dװ�õ�������
��2�����ڸ�ʵ�鷽������ش��������⣺
�������ȷ�Ӧ�����������NaCl�ⶨ�����Ӱ����___________����ƫ��������ƫ����������Ӱ������
��E���������ʢ�ŵ�ҩƷ��_______________����������_____________________________�����ʵ����û�и�װ�ã���ᵼ�²������NaHCO3����������______________����ƫ��������ƫ����������Ӱ������
������Ʒ����Ϊwg����Ӧ��C��Dװ�����ӵ������ֱ�Ϊm1g��m2g���ɴ˿�֪�������Na2CO3��10H2O����������Ϊ______���ú�w��m1��m2�Ĵ���ʽ��ʾ����
���𰸡����װ�������� ����װ���к��е�ˮ�����Ͷ�����̼��������� ƫ�� ��ʯ�� �����������еĶ�����̼��ˮ���� ƫ�� ![]() ����m1-
����m1-![]() m2����100%
m2����100%
��������
��NaCl��Na2CO310H2O��NaHCO3�Ļ�������ʱ��̼�����Ʒֽ�����̼���ơ�������̼��ˮ��̼���ƾ���ʧȥ�ᾧˮ����̼���ƣ���Ӧ�Ļ�ѧ����ʽΪ��2NaHCO3![]() Na2CO3+H2O��+CO2����Na2CO310H2O
Na2CO3+H2O��+CO2����Na2CO310H2O![]() Na2CO3+10H2O����������H2O(g)��CO2��Ӧ��C��D�зֱ����գ��ɸ����������֪Ӧ������ˮ�������ն�����̼����C�еĸ������ˮ����������CO2����D������(NaHCO3�ֽ������CO2������)�����NaHCO3��������C������(Na2CO310H2O�ֽ������H2O���Ѿ�֪����NaHCO3�ֽ������H2O������)�����Na2CO310H2O���������Ӷ����NaCl���������ݴ˷������
Na2CO3+10H2O����������H2O(g)��CO2��Ӧ��C��D�зֱ����գ��ɸ����������֪Ӧ������ˮ�������ն�����̼����C�еĸ������ˮ����������CO2����D������(NaHCO3�ֽ������CO2������)�����NaHCO3��������C������(Na2CO310H2O�ֽ������H2O���Ѿ�֪����NaHCO3�ֽ������H2O������)�����Na2CO310H2O���������Ӷ����NaCl���������ݴ˷������
(1)����ʵ��ԭ����֪��ʵ����Ҫͨ������Dװ���ڼ�ʯ�ҵ����أ��������ɵĶ�����̼��������ͨ������Cװ���ڵ����أ��������ɵ�ˮ����������Ӧ���ȼ���װ�õ������ԣ��ʴ�Ϊ�����װ�������ԣ�
��װ�����п����������к���ˮ�����Ͷ�����̼��Ӱ��ˮ�����Ͷ�����̼�����IJⶨ������K1��K2���رջ���K3��ʵ��ǰҪͨ�����������װ���к���ˮ�����Ͷ�����̼���������ʴ�Ϊ����ȥװ���е�ˮ�����Ͷ�����̼��
(2)�������ȷ�Ӧ���������ʹ��ˮ�����Ͷ�����̼�������ⶨ������С��̼���������ݶ�����̼���㣬��Na2CO310H2O�IJⶨ�Ǹ�������ˮ������������ģ������NaHCO3��Na2CO310H2O�ĺ�����ƫС����NaCl�IJⶨ�����ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
��E��������Ƿ�ֹ�������е�ˮ�����Ͷ�����̼Ӱ��ʵ��������˸������ʢ�ż�ʯ�ң���Ϊ��ʯ�������տ����е�ˮ�����Ͷ�����̼����û��Eװ�ã���ⶨ��̼�����Ƶ�����ƫ�ߣ��ʴ�Ϊ����ʯ�ң������������еĶ�����̼��ˮ������ƫ�ߣ�
�ۺ�NaCl��Na2CO310H2O��NaHCO3�Ļ�������ʱ��̼�����Ʒֽ�����̼���ơ�������̼��ˮ��̼���ƾ���ʧȥ�ᾧˮ����̼���ƣ���Ӧ�Ļ�ѧ����ʽΪ��2NaHCO3![]() Na2CO3+H2O��+CO2����Na2CO310H2O
Na2CO3+H2O��+CO2����Na2CO310H2O![]() Na2CO3+10H2O����Dװ�������ӵ�����Ϊ������̼����������̼�����Ʒֽ����ɵ�ˮ����������Ϊx��
Na2CO3+10H2O����Dװ�������ӵ�����Ϊ������̼����������̼�����Ʒֽ����ɵ�ˮ����������Ϊx��
2NaHCO3![]() Na2CO3+H2O + CO2��
Na2CO3+H2O + CO2��
18g 44g
x m2g
![]() =
=![]() �����x=
�����x=![]() m2g
m2g
װ��C���յ���ˮ����������̼�����Ʒֽ����ɵĺ�Na2CO310H2O�ֽ����ɵ�ˮ����Na2CO310H2O�ֽ����ɵ�ˮ����������=m1g -![]() m2g =(m1-
m2g =(m1-![]() m2)g����Na2CO310H2O������Ϊy��
m2)g����Na2CO310H2O������Ϊy��
Na![]() Na2CO3+10H2O
Na2CO3+10H2O
286g 180g
y (m1-![]() m2)g
m2)g
![]() =
=![]() ����ã�y=
����ã�y=![]() ��(m1-
��(m1-![]() m2)g������Na2CO3��10H2O����������=
m2)g������Na2CO3��10H2O����������=![]() ��100%=
��100%=![]() ��(m1-
��(m1-![]() m2)��100%���ʴ�Ϊ��
m2)��100%���ʴ�Ϊ��![]() ��(m1-
��(m1-![]() m2)��100%��
m2)��100%��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ�ִ��Բ��ϵ�ĥ�����ϣ�������������Լ21%����Ҫ�ɷ��������Ͻ𣬻�����ͭ���ơ�þ�����������ɸ÷����Ʊ����Ƚϸߵ�����������������������

�ش��������⣺
��1����������ʱ����Һ����Fe3����Fe2����Ni2�������ɣ���������Ҫ�ɷ���__________���������ܽ�����ӷ���ʽΪ__________________________________________��
��2����������ʱH2O2��������___________________������̼���Ƶ�Ŀ����__________________________________________��
��3������ͭ��ʱ����Ӧ�����ӷ���ʽΪ_____________________________________������Na2S����H2S��ͭ���ŵ���__________��
��4����֪����þ�������մ������н��У�NaF��ʵ���������ܹ��������Ϊ_____________________��
��5����֪������Ksp[Ni(OH)2]=2.0��10��15�������������������������������ҺpHԼΪ_______��Ni2���Ÿպó�����ȫ�����ӳ�����ȫ��Ũ����10��5 mol/L��lg2=0.30��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͬ�¶��£��ֱ�0.1molNa��Na2O��Na2O2��NaOH����ʢ��100mLˮ����ˮ���ܶ�Ϊ1g/cm3���ļס��ҡ��������ĸ��ձ��в��ָ���ԭ�¶ȣ���ס��ҡ����������ձ�����Һ������������С��ϵΪ
A. ��>��>��>�� B. ��>��>��>�� C. ��=��>��>�� D. ��>��=��>��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ϩ����Ȳ��������ԭ�ش���������:
��1���л���![]() ��ϵͳ����Ϊ______________��ʹ���ڴ���������������ȫ�⻯������������ϵͳ����Ϊ______________��
��ϵͳ����Ϊ______________��ʹ���ڴ���������������ȫ�⻯������������ϵͳ����Ϊ______________��
��2���л���![]() ��ϵͳ����Ϊ_____________��ʹ���ڴ���������������ȫ�⻯������������ϵͳ����Ϊ_____________��
��ϵͳ����Ϊ_____________��ʹ���ڴ���������������ȫ�⻯������������ϵͳ����Ϊ_____________��
��3���л���2-��-2-��ϩ�Ľṹ��ʽΪ_________��
��4��ijϩ���Ĵ���������2-��-4-�һ�-2-��ϩ����������ȷ������__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ӵ�������ֵΪNA������˵����ȷ����
A. ��״���£�2.24LCH3OH�����й��ۼ�����ĿΪ0.5NA
B. 1molNa2O2������CO2��ַ�Ӧ��ת�Ƶĵ�����Ϊ2 NA
C. 25��1LpH=12��Na2CO3��Һ�У���ˮ�����H+����ĿΪ0.01NA
D. 0.1molH2��0.1molI2���ܱ������г�ַ�Ӧ��HI��������Ϊ0.2NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1�����������У���˴Ź���������ֻ��һ�����շ����____(����ĸ��˫ѡ)��
A��CH3CH3 B��CH3COOH
C��CH3COOCH3 D��CH3OCH3
��2��������A��B�ķ���ʽ����C2H4Br2��A�ĺ˴Ź�������ͼ��ͼ��ʾ����A�Ľṹ��ʽΪ____����Ԥ��B�ĺ˴Ź���������Ӧ����____�����շ塣

��3��������C�и�ԭ����Ŀ��ΪN(C)��N(H)��N(O)=1��2��1���Ի�����C�����������ɵõ�ͼʾ������ͼ���������ʽΪ____����������к�����������ɵó��������Ϊ____(������)��ȷ��������C�Ĺ�����ʱ������Ϊ____(��С���û�С�)��Ҫ���к����������д��������C����������������ͭ����Һ��Ӧ�Ļ�ѧ����ʽ____________________________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л���ı�ʾ�������ֶ����������dz��õ��л���ı�ʾ������
��![]() ��
�� ��CH4 ��
��CH4 ��  ��
��![]()
��

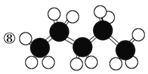

��
��1��������ʾ���������ڽṹ��ʽ��Ϊ__________��
���ڽṹʽ��Ϊ________��
���ڼ���ʽ��Ϊ________��
���ڱ���ģ�͵�Ϊ________��
�������ģ�͵�Ϊ________��
��2��д����ķ���ʽ��________��
��3��д�����й����ŵĵ���ʽ��________��________��
��4���ڵķ���ʽΪ________�����ʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪X��һ�־��й���ζ�ĺϳ����ϣ���ͼΪ�ϳ�X��ij�����̣�

��ʾ����![]() �������ձ�����Ϊ��COOH��
�������ձ�����Ϊ��COOH��
��D�IJ���������������һ�����ҵ�ʯ�ͻ���ˮƽ��
�����������Ϣ���ش��������⣺
(1)C�����й����ŵ�������__________��E�Ľṹ��ʽ��________��
(2)D+E��X�Ļ�ѧ��Ӧ����Ϊ________��Ӧ��
(3)����A��B��C��D��E��X���������У���Ϊͬϵ�����____________________��
(4)C��һ��ͬ���칹��F���Է���ˮ�ⷴӦ����F�Ľṹ��ʽΪ________�� ________��
(5)��ӦC��E��X�Ļ�ѧ����ʽΪ______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��ʵ������ȡ����ʱ�����в�������ȷ����________(����ĸ)��


A��װ�â��������ȡ��������
B����װ�â��ȥ�����е������Ȼ���
C����װ�â���ȡ����
D����װ�â���������
��2��ʵ������ȡ������������������˶������̡�Ũ�������Ҫ���Լ�____��_____��______��
��3����֪���������Ũ�����ڳ����·�Ӧ�ܲ�����������������ͼ��ʾ��ʵ��װ�����Ʊ��������������������֤��������ķ�Ӧ��ÿ�����߿��ʾһ����Ԫװ�ã������д������________(����ĸ)��

��4���������ж����壬�������β��������д��ʵ��������β�����������ӷ���ʽ___________��
��5��Ư��Һ���������ƣ��ͽ���飨���ᣩ���ܻ��ʹ�û���������ж���������ӷ���ʽ��____��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com