
����Ŀ���ס��Ҷ��Ƕ�Ԫ���廯�����32g�ķ�ĩ��������Ũ���Ტ���ȣ���ȫ�ܽ����ɫ��Һ�������Һ�м�������![]() ��Һ�����ˡ�ϴ�ӡ�����ó���
��Һ�����ˡ�ϴ�ӡ�����ó���![]() ����Һ���ٵμ�NaOH��Һ���ֳ�����ɫ������
����Һ���ٵμ�NaOH��Һ���ֳ�����ɫ������
���ҵĿ�ʯ��Ȼ���д����϶࣬��ȡһ�������ң�����ϡ����ʹ����ȫ�ܽ⣬��Һ��ΪA��B���ȷ֣���A�м�������NaOH��Һ�����ˡ�ϴ�ӡ����յõ�����ɫ����28g���������������ɫ�������Ԫ����ͬ����B�м���![]() ͭ�۳�ַ�Ӧ����ˡ�ϴ�ӡ�����ù���
ͭ�۳�ַ�Ӧ����ˡ�ϴ�ӡ�����ù���![]() ��
��
![]() д�����ɼ������ӵĽṹʾ��ͼ______��32g��������Ũ�����з�Ӧת�Ƶĵ�����Ϊ______��
д�����ɼ������ӵĽṹʾ��ͼ______��32g��������Ũ�����з�Ӧת�Ƶĵ�����Ϊ______��
![]() �ҵĻ�ѧʽ______��ϡ�����ܽ��ҵĻ�ѧ����ʽ______��
�ҵĻ�ѧʽ______��ϡ�����ܽ��ҵĻ�ѧ����ʽ______��
![]() ���������������г�����յ��������ͨ��һ����A��Һ�У��÷�Ӧ�����ӷ���ʽΪ______�����ʵ��֤���˲���Ӧ�����Һ�н���Ԫ�صĻ��ϼ�______��
���������������г�����յ��������ͨ��һ����A��Һ�У��÷�Ӧ�����ӷ���ʽΪ______�����ʵ��֤���˲���Ӧ�����Һ�н���Ԫ�صĻ��ϼ�______��
���𰸡�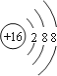
![]()
![]()
![]()
![]() ȡ��Ӧ�����Һ�������Թ��У���һ���м����ữ��
ȡ��Ӧ�����Һ�������Թ��У���һ���м����ữ��![]() ��Һ������Һ��ɫ��ԭ��
��Һ������Һ��ɫ��ԭ��![]() ���ӡ�����һ���м���KSCN��Һ��������Ѫ��ɫ��Һ����ԭ��
���ӡ�����һ���м���KSCN��Һ��������Ѫ��ɫ��Һ����ԭ��![]() ��������
��������
��������
��32g�ķ�ĩ��������Ũ���Ტ���ȣ���ȫ�ܽ����ɫ��Һ�������Һ�м�������![]() ��Һ�����ˡ�ϴ�ӡ�����ó���
��Һ�����ˡ�ϴ�ӡ�����ó���![]() ���ó���Ϊ���ᱵ�������ʵ���Ϊ��
���ó���Ϊ���ᱵ�������ʵ���Ϊ��![]() �����������غ��֪���к���
�����������غ��֪���к���![]() ԭ�ӣ���Һ���ٵμ�NaOH��Һ���ֳ�����ɫ����������ɫ����Ϊ������ͭ��˵�����к���Cu���ӣ�32g���к���ͭԪ�ص����ʵ���Ϊ��
ԭ�ӣ���Һ���ٵμ�NaOH��Һ���ֳ�����ɫ����������ɫ����Ϊ������ͭ��˵�����к���Cu���ӣ�32g���к���ͭԪ�ص����ʵ���Ϊ��![]() ����Ļ�ѧʽΪ��
����Ļ�ѧʽΪ��![]() ��
��
���ҵĿ�ʯ��Ȼ���д����϶࣬��ȡһ�����ң�����ϡ����ʹ��ȫ���ܽ⣬��Һ��ΪA��B���ȷݣ���A�м�����������������Һ�����ˡ�ϴ�ӡ����յú���ɫ����28g���ú���ɫ����Ϊ![]() ��������������Ԫ�ص����ʵ���Ϊ�
��������������Ԫ�ص����ʵ���Ϊ�![]() ������Ϊ�����Ӻ��������ӵĻ����������������ɫ��������Ԫ����ͬ�������к���Fe��OԪ�أ���B�м���
������Ϊ�����Ӻ��������ӵĻ����������������ɫ��������Ԫ����ͬ�������к���Fe��OԪ�أ���B�м���![]() ͭ�۳�ַ�Ӧ����ˡ�ϴ�ӡ������ʣ�����
ͭ�۳�ַ�Ӧ����ˡ�ϴ�ӡ������ʣ�����![]() ����Ӧ����ͭ�����ʵ���Ϊ��
����Ӧ����ͭ�����ʵ���Ϊ��![]() �����ݷ�Ӧ
�����ݷ�Ӧ![]() ��֪��
��֪��![]() ͭ��ȫ��Ӧ����
ͭ��ȫ��Ӧ����![]() �������������ᷴӦ���ɵ�Ϊ
�������������ᷴӦ���ɵ�Ϊ![]() ��
��![]() ������ƽ�����ϼ�Ϊ��
������ƽ�����ϼ�Ϊ��![]() �����ҵĻ�ѧʽΪ��
�����ҵĻ�ѧʽΪ��![]() ��
��
![]() ��Ϊ
��Ϊ![]() ������������Ϊ�����ӣ������ӽṹʾ��ͼΪ��
������������Ϊ�����ӣ������ӽṹʾ��ͼΪ�� ��
��![]() �����ʵ���Ϊ��
�����ʵ���Ϊ��![]() ��Ũ����������CuԪ�ش�
��Ũ����������CuԪ�ش�![]() ת����
ת����![]() �ۡ���Ԫ�ش�
�ۡ���Ԫ�ش�![]() ת����
ת����![]() �ۣ���Ӧ��ʧȥ���������ʵ���Ϊ��
�ۣ���Ӧ��ʧȥ���������ʵ���Ϊ��![]() ����Ӧת�Ƶ�������Ϊ
����Ӧת�Ƶ�������Ϊ![]() ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��![]() ��
��
![]() ���ݷ�����֪���ҵĻ�ѧʽΪ��
���ݷ�����֪���ҵĻ�ѧʽΪ��![]() ��ϡ�������ҷ�Ӧ�Ļ�ѧ����ʽΪ��
��ϡ�������ҷ�Ӧ�Ļ�ѧ����ʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��![]() ��
��
![]() �������������г�����յ��������Ϊ����������������ͨ��һ����A��Һ�У��÷�Ӧ�����ӷ���ʽ��
�������������г�����յ��������Ϊ����������������ͨ��һ����A��Һ�У��÷�Ӧ�����ӷ���ʽ��![]() ���÷�Ӧ����Һ�к����������ӣ������������ӵķ���Ϊ��ȡ��Ӧ�����Һ�������Թ��У���һ���м����ữ��
���÷�Ӧ����Һ�к����������ӣ������������ӵķ���Ϊ��ȡ��Ӧ�����Һ�������Թ��У���һ���м����ữ��![]() ��Һ������ɫ������
��Һ������ɫ������![]() ����������һ���м���KSCN��Һ��������Ѫ��ɫ��Һ������
����������һ���м���KSCN��Һ��������Ѫ��ɫ��Һ������![]() �������ʴ�Ϊ��
�������ʴ�Ϊ��![]() ��ȡ��Ӧ�����Һ�������Թ��У���һ���м����ữ��
��ȡ��Ӧ�����Һ�������Թ��У���һ���м����ữ��![]() ��Һ������ɫ������
��Һ������ɫ������![]() ����������һ���м���KSCN��Һ��������Ѫ��ɫ��Һ������
����������һ���м���KSCN��Һ��������Ѫ��ɫ��Һ������![]() ��������32g�ķ�ĩ��������Ũ���Ტ���ȣ���ȫ�ܽ����ɫ��Һ�������Һ�м�������
��������32g�ķ�ĩ��������Ũ���Ტ���ȣ���ȫ�ܽ����ɫ��Һ�������Һ�м�������![]() ��Һ�����ˡ�ϴ�ӡ�����ó���
��Һ�����ˡ�ϴ�ӡ�����ó���![]() ���ó���Ϊ���ᱵ�������ʵ���Ϊ��
���ó���Ϊ���ᱵ�������ʵ���Ϊ��![]() �����������غ��֪���к���
�����������غ��֪���к���![]() ԭ�ӣ���Һ���ٵμ�NaOH��Һ���ֳ�����ɫ����������ɫ����Ϊ������ͭ��˵�����к���Cu���ӣ�32g���к���ͭԪ�ص����ʵ���Ϊ��
ԭ�ӣ���Һ���ٵμ�NaOH��Һ���ֳ�����ɫ����������ɫ����Ϊ������ͭ��˵�����к���Cu���ӣ�32g���к���ͭԪ�ص����ʵ���Ϊ��![]() ����Ļ�ѧʽΪ��
����Ļ�ѧʽΪ��![]() �����ҵĿ�ʯ��Ȼ���д����϶࣬��ȡһ�����ң�����ϡ����ʹ��ȫ���ܽ⣬��Һ��ΪA��B���ȷݣ���A�м�����������������Һ�����ˡ�ϴ�ӡ����յú���ɫ����28g���ú���ɫ����Ϊ
�����ҵĿ�ʯ��Ȼ���д����϶࣬��ȡһ�����ң�����ϡ����ʹ��ȫ���ܽ⣬��Һ��ΪA��B���ȷݣ���A�м�����������������Һ�����ˡ�ϴ�ӡ����յú���ɫ����28g���ú���ɫ����Ϊ![]() ��������������Ԫ�ص����ʵ���Ϊ�
��������������Ԫ�ص����ʵ���Ϊ�![]() ������Ϊ�����Ӻ��������ӵĻ����������������ɫ��������Ԫ����ͬ�������к���Fe��OԪ�أ���B�м���
������Ϊ�����Ӻ��������ӵĻ����������������ɫ��������Ԫ����ͬ�������к���Fe��OԪ�أ���B�м���![]() ͭ�۳�ַ�Ӧ����ˡ�ϴ�ӡ������ʣ�����
ͭ�۳�ַ�Ӧ����ˡ�ϴ�ӡ������ʣ�����![]() ����Ӧ����ͭ�����ʵ���Ϊ��
����Ӧ����ͭ�����ʵ���Ϊ��![]() �����ݷ�Ӧ
�����ݷ�Ӧ![]() ��֪��
��֪��![]() ͭ��ȫ��Ӧ����
ͭ��ȫ��Ӧ����![]() �������������ᷴӦ���ɵ�Ϊ
�������������ᷴӦ���ɵ�Ϊ![]() ��
��![]() ������ƽ�����ϼ�Ϊ��
������ƽ�����ϼ�Ϊ��![]() �����ҵĻ�ѧʽΪ��
�����ҵĻ�ѧʽΪ��![]() ���ݴ˷������
���ݴ˷������


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ռ�ʵ�������칬һ�����Ĺ���ϵͳ������������ȼ�ϵ�أ�RFC����RFC��һ�ֽ�ˮ��⼼��������ȼ�ϵ�ؼ������ϵĿɳ���ء���ͼΪRFC����ԭ��ʾ��ͼ���й�˵����ȷ���ǣ� ��

A. ����0.8mol����ת��ʱ��b������4.48LO2
B. Ϊ�����ӵ����Կ��Խ���������е�ˮ��ΪNaOH��Һ
C. d���Ϸ����ĵ缫��Ӧ�ǣ�2H+ +2e��=H2
D. c���Ͻ���������Ӧ��A���е�H+����ͨ����Ĥ����B
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ�ĸ������ʾһ�ַ�Ӧ���������(ijЩ�����Ѿ���ȥ)�����г�����A��C��DΪ��ɫ���壬C��ʹʪ��ĺ�ɫʯ����ֽ������
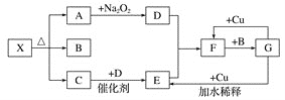
��1��д������X�Ļ�ѧʽ��__��
��2��д�����б仯�Ļ�ѧ����ʽ��
��A��D��___��
��G��E��___��
��F��G��___��
��3��ʵ��������ü��ȹ�������ķ�����ȡ����C����д����ѧ����ʽ��___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����( )
��֪��2H2(g)��O2(g)=2H2O(l) ��H����571.6 kJmol��1
2CH3OH(l)��3O2(g)=2CO2(g)��4H2O(l) ��H����1452 kJmol��1
H��(aq)��OH��(aq)=H2O(l) ��H����57.3 kJmol��1
A.H2(g)��ȼ����Ϊ571.6 kJmol��1
B.ͬ������H2(g)��CH3OH(l)��ȫȼ�գ�H2(g)�ų���������
C.H2SO4(aq)��Ba(OH)2(aq)=BaSO4(s)��H2O(l) ��H����57.3 kJmol��1
D.3H2(g)��CO2(g)=CH3OH(l)��H2O(l) ��H����135.9 kJmol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25��ʱ����Ԫ��H3PO3��pKa1��pKa2(pK����lgK)����Ϊ1.30��6.60����ˮ��pKbΪ4.75������ʱ������ָ����Һ�������ʵ���Ũ�ȹ�ϵ��ȷ����(����)
A.0.1 mol��L��1NH4H2PO3��Һ�У�c(H3PO3)>c(NH3��H2O)>c(H��)>c(OH��)
B.0.1 mol��L��1H3PO3��Һ��NaOH��Һ�ζ���pH��6.60:c(H2PO3-)��c(HPO32-)
C.0.1 mol��L��1H3PO3��Һ�ð�ˮ�ζ���pH��7.0:c(NH4+)��c(H2PO3- )��c(HPO32-)
D.0.4 mol��L��1��ˮ��0.2 mol��L��1NaH2PO3��������(����仯�ɺ���)��c(NH3��H2O)<c(H2PO3-)��2c(H3PO3)��0.1 mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й�ͬ���칹����Ŀ�������У���ȷ����
A. ����ϩ��������ȫ�ӳɵIJ����һ��ȡ������5��
B. �����ʵ����������������ڹ��������·�Ӧ�õ�9�ֲ���
C. ��֪���ȱ���3��ͬ���칹�壬�����ȱ���ͬ���칹�����ĿΪ6��
D. �ױ������ϵ�һ����ԭ�ӱ���4��̼ԭ�ӵ����ȡ�������ò�����12��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ᶼ����Ҫ�Ĺ�ҵԭ�ϡ�
��1����״���£���500L��������ˮ�γ�1L��ˮ����˰�ˮ�����ʵ���Ũ��Ϊ____mol��L-1��������λ��Ч���֣�����ҵ�ϳ��ù�����ˮ���ն������÷�Ӧ�Ļ�ѧ����ʽΪ____��
��2�����������ǹ�ҵ�����кϳ��������Ҫ;�����ϳɵĵ�һ���ǽ����Ϳ����Ļ����ͨ�����ȵIJ���Ͻ������ںϽ����Ĵ��£�����������һ���������÷�Ӧ�Ļ�ѧ����ʽΪ____��
���з�Ӧ�еİ��백�������еİ�������ͬ����____��
A.2Na+2NH3=2NaNH2+H2�� B.2NH3+3CuO=3Cu+N2+3H2O
C.4NH3+6NO=5N2+6H2O D.HNO3+NH3=NH4NO3
��ҵ�е�β��������ֻ��NO��NO2�����ռ�������գ���Ӧ�����ӷ���ʽΪ2NO2+2OH-=NO2-+NO3-+H2O��NO+NO2+2OH-=____+H2O����ƽ�÷���ʽ����
��3����27.2gCu��Cu2O�Ļ�����м���ijŨ�ȵ�ϡHNO3500mL����Ӧ�����в���������ֻ��NO��������ȫ�ܽ����������Һ������������ֻ��Cu2+���м���1L1mol��L-1��NaOH��Һʹ��������ǡ����ȫ��������ʱ��Һ�����ԣ����ó�������Ϊ39.2g��
��Cu��ϡHNO3��Ӧ�����ӷ���ʽΪ____��
��Cu��Cu2O�����ʵ���֮��Ϊ____��
��HNO3�����ʵ���Ũ��Ϊ____mol��L-1��
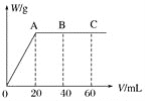
��4����H2SO4��HNO3�Ļ����Һ20mL������0.25molL-1Ba��OH��2��Һʱ�����ɳ���������w��g����Ba��OH��2��Һ�����V��mL���Ĺ�ϵ��ͼ��ʾ��C����Һ�����ԣ�����ԭ���Һ��H2SO4�����ʵ���Ũ��Ϊ____mol��L-1��HNO3�����ʵ���Ũ��Ϊ____mol��L-1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��ͨ���ǻ���̼̼˫������ʱ���ȶ����������б仯�� 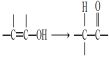 ����������ͼ��ʾ��ת����ϵ���ش����⣺
����������ͼ��ʾ��ת����ϵ���ش����⣺
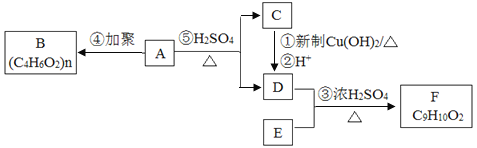
��1��A�Ļ�ѧʽ��___����������������___�������ƣ���
��2��B�Ľṹ��ʽ��_____��
��3���ٵĻ�ѧ����ʽ��____��
��4��F�Ƿ����廯�����ұ�����ֻ��һ���������۵Ļ�ѧ����ʽ��____��
��5����ɫ��ѧ�У����������ԭ�Ӿ�������ԭ��������100%��������Ӧ����������ԭ�Ӿ�����ԭ�����__��ѡ����ĸ����
a���� b���� c���� d����
��6��G��F��ͬ���칹�壬�й�G�����������ܷ���ˮ�⣬�ڱ�����������ȡ�������۱�����һ�������2�֡��ݴ��Ʋ�G�Ľṹ��ʽ�����ǣ�д������һ�֣�__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������кϳ�·�ߣ�
CH2=CH-CH=CH2![]() A��B��C��
A��B��C��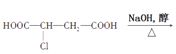 D
D![]() E
E![]() CH3OOC-CH=CH-COOCH3
CH3OOC-CH=CH-COOCH3
��B����ӦΪ�����������ʣ� ��
A.![]() B.
B.![]()
C.![]() D.
D.![]()
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com