
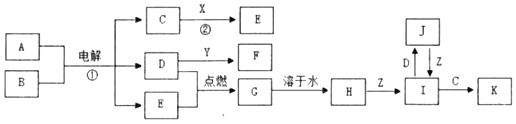
ЎҫМвДҝЎҝАыУГФӘЛШөД»ҜәПјЫНЖІвОпЦКөДРФЦККЗ»ҜС§СРҫҝөДЦШТӘКЦ¶ОЎЈНјКЗБтФӘЛШөДіЈјы»ҜәПјЫУлІҝ·ЦОпЦКАаұрөД¶ФУҰ№ШПөЎЈ
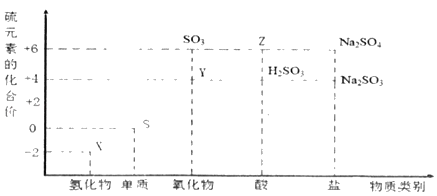
(1)ҙУБтФӘЛШ»ҜәПјЫұд»ҜөДҪЗ¶И·ЦОцЈ¬НјЦРјИУРСх»ҜРФУЦУР»№ФӯРФөДСх»ҜОпОӘ_____(Мо»ҜС§КҪ)ЎЈ
(2)Ҫ«XУлY»мәПҝЙЙъіЙөӯ»ЖЙ«№ММеЈ¬ёГ·ҙУҰөД»ҜС§·ҪіМКҪОӘ_____ЎЈ
(3)ZөДЕЁИЬТәУлМјФЪТ»¶ЁМхјюПВҝЙТФ·ўЙъ·ҙУҰЈ¬МеПЦБЛZөД_____РФЎЈ
(4)РҙіцБтУлЕЁБтЛбФЪјУИИМхјюПВ·ҙУҰөД»ҜС§·ҪіМКҪ_____ЎЈ
(5)Na2S2O3КЗЦШТӘөД»Ҝ№ӨФӯБПЎЈҙУСх»Ҝ»№Фӯ·ҙУҰөДҪЗ¶И·ЦОцЈ¬ПВБРЦЖұёNa2S2O3өД·Ҫ·ЁАнВЫЙПҝЙРРөДКЗ_____(МоЧЦДё)ЎЈ
aЈ®Na2SO3+S bЈ®Na2S+S cЈ®SO2+Na2SO4 dЈ®Na2SO3+Na2SO4
Ўҫҙр°ёЎҝSO2 2H2S+SO2ЈҪ3SЎэ+2H2O ЗҝСх»Ҝ S+2H2SO4(ЕЁ)![]() 3SO2Ўь+2H2O a
3SO2Ўь+2H2O a
ЎҫҪвОцЎҝ
ЈЁ1Ј©ФӘЛШҙҰУЪЦРјдјЫМ¬Ј¬јИҫЯУРСх»ҜРФУЦУР»№ФӯРФЈ»
ЈЁ2Ј©XОӘH2SЈ¬YОӘSO2Ј¬·ўЙъ2H2S+SO2=3SЎэ+2H2OЈ»
ЈЁ3Ј©ZОӘЕЁБтЛбЈ¬CУлЕЁБтЛб·ҙУҰЙъіЙ¶юСх»ҜМјЎў¶юСх»ҜБтәНЛ®Ј»
ЈЁ4Ј©БтУлЕЁБтЛбФЪјУИИМхјюПВ·ҙУҰЙъіЙ¶юСх»ҜБтәНЛ®Ј»
ЈЁ5Ј©ЦЖұёNa2S2O3КұЈ¬·ҙУҰОпЦРSФӘЛШөД»ҜәПјЫҙуУЪ+2ЎўРЎУЪ+2ЎЈ
(1)ҙҰУЪЦРјдјЫМ¬өДФӘЛШҫЯУРСх»ҜРФәН»№ФӯРФЈ¬ФтНјЦРјИУРСх»ҜРФУЦУР»№ФӯРФөД»ҜәПОпУРSO2ЎўH2SO3ЎўNa2SO3Ј¬Сх»ҜОпОӘSO2Ј¬
№Кҙр°ёОӘЈәSO2Ј»
(2)XОӘH2SЈ¬YОӘSO2Ј¬XОӘ»№ФӯјБЈ¬YОӘСх»ҜјБЈ¬ФтСх»ҜјБУл»№ФӯјБөДОпЦКөДБҝЦ®ұИОӘ1Јә2Ј¬·ўЙъ·ҙУҰөД»ҜС§·ҪіМКҪЈә2H2S+SO2ЈҪ3SЎэ+2H2OЈ¬
№Кҙр°ёОӘЈә2H2S+SO2ЈҪ3SЎэ+2H2OЈ»
(3)ZОӘЕЁБтЛбЈ¬CУлЕЁБтЛб·ҙУҰЙъіЙ¶юСх»ҜМјЎў¶юСх»ҜБтәНЛ®Ј¬МеПЦБЛЕЁБтЛбөДЗҝСх»ҜРФЈ¬
№Кҙр°ёОӘЈәЗҝСх»ҜЈ»
(4)БтУлЕЁБтЛбФЪјУИИМхјюПВ·ҙУҰЙъіЙ¶юСх»ҜБтәНЛ®Ј¬·ҙУҰөД»ҜС§·ҪіМКҪЈәS+2H2SO4(ЕЁ)![]() 3SO2Ўь+2H2OЈ¬
3SO2Ўь+2H2OЈ¬
№Кҙр°ёОӘЈәS+2H2SO4(ЕЁ)![]() 3SO2Ўь+2H2OЈ»
3SO2Ўь+2H2OЈ»
(5)ЦЖұёNa2S2O3КұЈ¬·ҙУҰОпЦРSФӘЛШөД»ҜәПјЫҙуУЪ+2ЎўРЎУЪ+2Ј¬Ц»УРa·ыәПЈ¬
№Кҙр°ёОӘЈәaЎЈ


 ҪМС§Б·РВН¬ІҪБ·П°ПөБРҙр°ё
ҪМС§Б·РВН¬ІҪБ·П°ПөБРҙр°ё ҝОЗ°ҝОәуН¬ІҪБ·П°ПөБРҙр°ё
ҝОЗ°ҝОәуН¬ІҪБ·П°ПөБРҙр°ё
| Дкј¶ | ёЯЦРҝОіМ | Дкј¶ | іхЦРҝОіМ |
| ёЯТ» | ёЯТ»Гв·СҝОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СҝОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СҝОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СҝОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СҝОіМНЖјцЈЎ | іхИэ | іхИэГв·СҝОіМНЖјцЈЎ |
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝПВБРІ»ДЬУГАХЙіМШБРФӯАнҪвКНөДКЗЈЁ Ј©
A. Fe(SCN)3ИЬТәЦРјУИл№ММеKSCNәуСХЙ«ұдЙо
B. ЧШәмЙ«NO2јУС№әуСХЙ«ПИұдЙоәуұдЗі
C. SO2ҙЯ»ҜСх»ҜіЙSO3өД·ҙУҰЈ¬НщНщРиТӘК№УГҙЯ»ҜјБ
D. ёЯС№ұИіЈС№УРАыУЪNH3өДәПіЙ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝИзНјЛщКҫЈ¬Ҫ«Мъ°фәНКҜД«°фІеИл1 L 1 mol/LКіСОЛ®ЦРЎЈПВБРЛө·ЁХэИ·өДКЗЈЁ Ј©
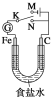
A. ИфөзјьKУлNБ¬ҪУЈ¬Мъұ»ұЈ»ӨІ»»бёҜКҙ
B. ИфөзјьKУлNБ¬ҪУЈ¬Хэј«·ҙУҰКҪКЗ4OHЈӯЈӯ4eЈӯ===2H2OЈ«O2Ўь
C. ИфөзјьKУлMБ¬ҪУЈ¬Ҫ«КҜД«°ф»»іЙНӯ°фЈ¬ҝЙКөПЦМъ°фЙП¶ЖНӯ
D. ИфөзјьKУлMБ¬ҪУЈ¬өұБҪј«№ІІъЙъ28 L(ұкЧјЧҙҝц)ЖшМеКұЈ¬ЙъіЙБЛ1 mol NaOH
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝКТОВПВЈ¬ёК°ұЛбФЪЛ®ИЬТәЦРЦчТӘТФ Ўў
Ўў әН
әН![]() ИэЦЦОўБЈРОКҪҙжФЪЈ¬КөСйІвөГІ»Н¬pHёК°ұЛбИЬТәЦРёчіЙ·Ц·ЦІј·ЦКэҰДУлpH№ШПөИзНјЛщКҫЎЈПВБРЛө·ЁХэИ·өДКЗЈЁ Ј©
ИэЦЦОўБЈРОКҪҙжФЪЈ¬КөСйІвөГІ»Н¬pHёК°ұЛбИЬТәЦРёчіЙ·Ц·ЦІј·ЦКэҰДУлpH№ШПөИзНјЛщКҫЎЈПВБРЛө·ЁХэИ·өДКЗЈЁ Ј©
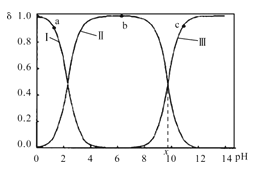
A.aөгИЬТәЦРЈ¬Л®өДөзАліМ¶ИҙуУЪbөг
B.cөгИЬТәЦРЈ¬c(![]() )>c(
)>c( )
)
C.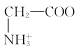 +H2O
+H2O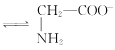 +H3O+өДЖҪәвіЈКэОӘx
+H3O+өДЖҪәвіЈКэОӘx
D.aөгИЬТәЦРЈ¬ҙжФЪ№ШПөКҪЈәc(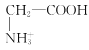 )+c(H+)=c(
)+c(H+)=c(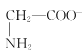 )+c(OH-)
)+c(OH-)
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝУР№ШОпЦКЦ®јдөДЧӘ»Ҝ№ШПөИзНјЈ¬ДіР©·ҙУҰөДІҝ·ЦОпЦКәН·ҙУҰМхјюұ»ВФИҘЎЈТСЦӘXЎўYЎўZКЗИХіЈЙъ»оЦРіЈјыҪрКфөҘЦКЈ¬XУЙөШҝЗЦРә¬БҝЧоёЯөДҪрКфФӘЛШЧйіЙЎЈAКЗәЈЛ®ЦРә¬БҝЧо¶аөДСОЈ¬BКЗіЈјыөДОЮЙ«ТәМеЈ¬DЎўEКЗіЈјыЖшМ¬·ЗҪрКфөҘЦКЈ¬ЖдЦРDіК»ЖВМЙ«ЎЈFөДПЎИЬТәіКА¶Й«ЎЈ
Зл»ШҙрПВБРОКМвЈә
(1)ТФ·ҙУҰўЩОӘФӯАнөД№ӨТөұ»іЖОӘ_____№ӨТөЎЈ
(2)KөД»ҜС§КҪОӘ_____ЎЈ
(3)·ҙУҰўЪөДАлЧУ·ҪіМКҪОӘ_____ЎЈ
(4)JәНY·ҙУҰөД»ҜС§·ҪіМКҪОӘ_____ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝПВБРЛө·ЁХэИ·өДКЗ
A.![]() Ул
Ул![]() »ҘОӘН¬О»ЛШ
»ҘОӘН¬О»ЛШ
B.O2әНO3»ҘОӘН¬ЛШТмРОМеЈ¬БҪХЯЦ®јдІ»ДЬПа»ҘЧӘ»Ҝ
C.ТТҙјәНјЧГС»ҘОӘН¬·ЦТм№№МеЈ¬ҝЙУГҪрКфДЖјшұр
D.![]() өДТ»ВИҙъОпЦ»УР2ЦЦ(І»ҝјВЗБўМеТм№№)
өДТ»ВИҙъОпЦ»УР2ЦЦ(І»ҝјВЗБўМеТм№№)
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝҝЙДж·ҙУҰ3A(g)![]() 3B(Јҝ)+C(Јҝ)ЎчH>0Ј¬ҙпөҪ»ҜС§ЖҪәвәуЈ¬ҪшРРПВБРІЩЧчЎЈ
3B(Јҝ)+C(Јҝ)ЎчH>0Ј¬ҙпөҪ»ҜС§ЖҪәвәуЈ¬ҪшРРПВБРІЩЧчЎЈ
(1)ЙэёЯОВ¶ИЈ¬УГЎ°ұдҙуЎұЎ°ұдРЎЎұЎ°І»ұдЎұ»тЎ°ОЮ·ЁИ·¶ЁЎұМоҝХЎЈ
ўЩИфBЎўC¶јКЗЖшМеЈ¬ЖшМеөДЖҪҫщПа¶Ф·ЦЧУЦКБҝ______Ј»
ўЪИфBЎўC¶јІ»КЗЖшМеЈ¬ЖшМеөДЖҪҫщПа¶Ф·ЦЧУЦКБҝ______Ј»
ўЫИфBКЗЖшМеЈ¬CІ»КЗЖшМеЈ¬ЖшМеөДЖҪҫщПа¶Ф·ЦЧУЦКБҝ______Ј»
(2)Из№ыЖҪәвәуОВ¶ИұЈіЦІ»ұдЈ¬Ҫ«ИЭЖчМе»эФцҙуТ»ұ¶Ј¬РВЖҪәвКұAөДЕЁ¶ИКЗФӯАҙөД50ЈҘЈ¬ЕР¶ПBөДЧҙМ¬КЗ__________М¬Ј¬CөДЧҙМ¬КЗ__________М¬ЎЈ
(3)ДіГЬұХИЭЖчЦР·ЕИлТ»¶ЁБҝөДNO2Ј¬·ўЙъ·ҙУҰ2NO2![]() N2O4ЈЁХэ·ҙУҰ·ЕИИЈ©Ј¬ҙпЖҪәвәуЈ¬Иф·ЦұрөҘ¶АёДұдПВБРМхјюЈ¬ЦШРВҙпөҪЖҪәвәуЈ¬ДЬК№»мәНЖшМеЖҪҫщ·ЦЧУБҝФцҙуөДКЗ_________ЎЈ
N2O4ЈЁХэ·ҙУҰ·ЕИИЈ©Ј¬ҙпЖҪәвәуЈ¬Иф·ЦұрөҘ¶АёДұдПВБРМхјюЈ¬ЦШРВҙпөҪЖҪәвәуЈ¬ДЬК№»мәНЖшМеЖҪҫщ·ЦЧУБҝФцҙуөДКЗ_________ЎЈ
A.НЁИлN2 B.НЁИлNO2 C.НЁИлN2O4 D.ЙэёЯОВ¶И
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝУГөзКҜЦЖұёөДТТИІЖшМеЦРіЈ»мУРЙЩБҝH2SЖшМеЎЈЗлУГНјЦРТЗЖчәНТ©Ж·ЧйіЙТ»МЧЦЖұёЎўҫ»»ҜТТИІөДЧ°ЦГЈ¬ІўҝЙНЁ№эІв¶ЁТТИІөДБҝЈ¬ҙУ¶шјЖЛгөзКҜҙҝ¶ИЎЈ
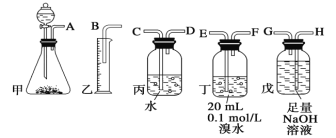
(1)өзКҜЦЖұёТТИІЖшМеөД»ҜС§·ҪіМКҪ____________Ј»
(2)ҪшРРКөСйКұЈ¬ЛщЦЖЖшМеҙУЧуПтУТБчЈ¬ТЗЖчөДХэИ·Б¬ҪУЛіРтКЗ____________(МоҪУҝЪЧЦДё)Ј»
(3)ОӘБЛК№КөСйЦРЖшБчЖҪОИЈ¬јЧЦР·ЦТәВ©¶·АпөДТәМеНЁіЈУГ_______________Ј»
(4)ИфФЪұкЧјЧҙҝцПВдеЛ®УлТТИІНкИ«·ҙУҰЙъіЙC2H2Br4Ј¬ТСЦӘіЖИЎөзКҜm gЈ¬ІвөГБҝНІДЪТәМеМе»эV mLЈ¬ФтөзКҜҙҝ¶ИҝЙұнКҫОӘ____________Ј»
(5)ИфГ»УРЧ°ЦГОмЈ¬Ів¶ЁҪб№ыҪ«»б______(МоЎ°Ж«ёЯЎұЎўЎ°Ж«өНЎұ»тЎ°І»ұдЎұ) Ј¬АнУЙКЗЈЁ·ҪіМКҪұнКҫЈ©____Ј»
(6)ёЙФпТТИІҝЙУГ_____________ЈЁМоСЎПоЈ©Ј»
A. 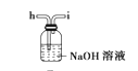
B.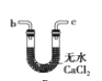
C.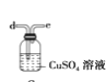
D.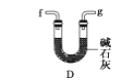
(7)ОӘБЛМҪҫҝТТИІУлHBr·ўЙъјУіЙ·ҙУҰәуөДУР№ШІъОпЈ¬ҪшРРТФПВКөСйЈәҙҝҫ»ТТИІЖш ![]() »мәПТә
»мәПТә ![]() УР»ъ»мәПОпўс
УР»ъ»мәПОпўс ![]() »мәПТә
»мәПТә ![]() УР»ъ»мәПОпўт
УР»ъ»мәПОпўт
ўЩІЩЧчbөДГыіЖКЗ________Ј»
ўЪУР»ъ»мәПОпўсҝЙДЬә¬УРөДОпЦККЗ________(РҙҪб№№јтКҪ)ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝКөСйКТУыУГNaOH№ММеЕдЦЖ1.0 mol/LөДNaOHИЬТә480 mLЈә
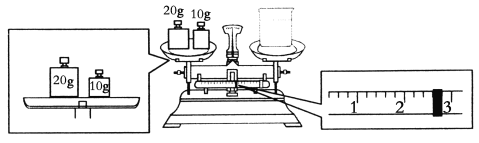
(1)ЕдЦЖКұЈ¬ұШРлК№УГөДІЈБ§ТЗЖчУРҪәН·өО№ЬЎўЙХұӯЎўІЈБ§°фЎў________ЎЈ
(2)ТӘНкіЙұҫКөСйёГН¬С§УҰіЖіцNaOH________gЎЈ
(3)ДіН¬С§УыіЖБҝNaOHөДЦКБҝЈ¬ЛыПИУГНРЕММмЖҪіЖБҝЙХұӯөДЦКБҝЈ¬МмЖҪЖҪәвәуөДЧҙМ¬ИзНјЈ¬ЙХұӯөДКөјКЦКБҝОӘ________gЎЈ
(4)К№УГИЭБҝЖҝЗ°ұШРлҪшРРөДТ»ІҪІЩЧчКЗ________ЎЈ
(5)ФЪЕдЦЖ№эіМЦРЈ¬ЖдЛыІЩЧч¶јКЗХэИ·өДЈ¬ПВБРІЩЧч»бТэЖрОуІоЖ«ёЯөДКЗ________ЎЈ
ўЩГ»УРПҙөУЙХұӯәНІЈБ§°ф
ўЪЧӘТЖИЬТәКұІ»ЙчУРЙЩБҝИчөҪИЭБҝЖҝНвГж
ўЫИЭБҝЖҝІ»ёЙФпЈ¬ә¬УРЙЩБҝХфБуЛ®
ўЬ¶ЁИЭКұё©КУҝМ¶ИПЯ
ўЭОҙАдИҙөҪКТОВҫНҪ«ИЬТәЧӘТЖөҪИЭБҝЖҝІў¶ЁИЭ
ўЮ¶ЁИЭәуИыЙПЖҝИы·ҙёҙТЎФИЈ¬ҫІЦГәуЈ¬ТәГжөНУЪҝМ¶ИПЯЈ¬ФЩјУЛ®ЦБҝМ¶ИПЯ
Ійҝҙҙр°ёәНҪвОц>>
№ъјКѧУУЕСЎ - Б·П°ІбБРұн - КФМвБРұн
әюұұКЎ»ҘБӘНшОҘ·ЁәНІ»БјРЕПўҫЩұЁЖҪМЁ | НшЙПУРәҰРЕПўҫЩұЁЧЁЗш | өзРЕХ©ЖӯҫЩұЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРәҰРЕПўҫЩұЁЧЁЗш | ЙжЖуЗЦИЁҫЩұЁЧЁЗш
ОҘ·ЁәНІ»БјРЕПўҫЩұЁөз»°Јә027-86699610 ҫЩұЁУКПдЈә58377363@163.com