
����Ŀ����1��ijʵ������Ҫ����480 mL 0.10 mol/L Na2CO3��Һ��

�����貣�������У����������ձ���100mL��Ͳ��______________________��
��ʵ��ʱͼ����ʾ�������Ⱥ�˳��Ϊ________________(����)
��2��������������Ϊ98�G���ܶ�Ϊ1.84g/mL��Ũ����������500mL 0.2mol/L ��ϡ���ᡣ�ɹ�ѡ��������У��ٲ����� ����ƿ ���ձ� �ܽ�ͷ�ι� ����Ͳ ������ƿ ��������ƽ ��ҩ�ס�����������⣺
�����������У�������ϡ����ʱ����Ҫ�õ�����_______________________������ţ�
�ھ������㣬��Ҫ��ȡŨ��������Ϊ_______�����Т�10mL ��50mL ��100mL ���ֹ���������ѡ�õĹ����_________������ţ�
�����Ʋ����ɷֽ�����¼�����������ȷ�IJ���˳����____________________________
A�� ������ƿ��ע����������ˮ������Ƿ�©ˮ
B�� ����������ˮϴ���ձ���������������Һע������ƿ�����ظ���������
C�� ������ȴ��ϡ����ע���Ѽ�鲻©ˮ��100mL����ƿ��
D�� ���ݼ��㣬����Ͳ��ȡһ�������Ũ����
E�� ��Ũ�������ձ�������ע��ʢ������ˮ��С�ձ��У��������ò���������
F�� ��������ƿ���ӣ���ҡ��
G�� �ý�ͷ�ιܵμ�����ˮ��ʹ��Һ����ǡ����̶�����
H�� ����������ƿ��С�ĵؼ�����ˮ��ʹҺ��ӽ��̶���1-2 cm��
�������ƹ����У����в����������______����ʹ������ҺŨ��ƫ�ߵ���______������ţ�
a.ϴ����ȡŨ��������Ͳ������ϴ��Һת��������ƿ��
b.δ��ϡ�ͺ��������Һ��ȴ�����¾�ת�Ƶ�����ƿ��
c.����ʱ�����ӿ̶���
d.��Ũ����ֱ�ӵ����ձ��������ձ���ע������ˮ��ϡ��Ũ����
e.����ʱ��������ˮ�����̶��ߣ����ý�ͷ�ι�����
f.����ҡ�Ⱥ���Һ����ڿ̶��ߣ����ý�ͷ�ιܼ�����ˮ���̶���
��������ƿ��ת����Һʱ������Һ����������ƿ���潫����������ҺŨ��__________���ƫ�ߡ�����ƫ�͡�����Ӱ�족�����������������㽫��δ���_______________��
���𰸡� 500mL����ƿ����ͷ�ι� �ڢܢۢݢ٢� �ڢߢ� 5.4mL �� ADECBHGF abcdef abc ƫ�� ϴ������ƿ����������
����������1��ijʵ������Ҫ����480 mL 0.10 mol/L Na2CO3��Һ���ٻ�Ҫ��500mL����ƿ����ͷ�ιܣ������Ʋ�������ȡ��ϡ�͡���Һ��ϴ�ӡ����ݡ�ҡ�ȣ�ʵ��ʱͼ����ʾ�������Ⱥ�˳��Ϊ�ڢܢۢݢ٢�����2�����Ʋ�������ȡ��ϡ�͡���Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ������Ͳ��ȡŨ������Һ���ձ���ϡ�ͣ���ȴ��ת�Ƶ�500mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ�������в���������Ͳ����ͷ�ιܡ�С�ձ���500ml����ƿ�������ò�������������ƿ��������ƽ��ҩ�ף��ʴ�Ϊ���ڢߢࣻ�ھ������㣬��Ҫ��ȡŨ��������ΪŨ��������ʵ���Ũ��Ϊ��103��w/M=103��1.84��98%/98mol��L��1=18.4mol��L��1������ҪŨ������Һ�����ΪV��0.2mol��L��1��0.5L=18.4mol��L��1��V��V=0.0054L=5.4mL����Ͳ���ݻ�Ӧ�Դ��ڻ������ȡ��Һ���������ѡ�٣�����Ũ��Һ����һ�����ʵ�Ũ����Һһ�㲽��Ϊ�����㡢��ȡ���ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ������ǩ������ȷ�IJ�������Ϊ��ADECBHGF����a.����ϴ����ȡŨ��������Ͳ���罫ϴ��Һת��������ƿ�У�����Ũ��ƫ�ߣ�b.���ϡ�ͺ��������Һ��ȴ��������ת�Ƶ�����ƿ�У���������Ũ��ƫ�ߣ�c.����ʱ��Ҫƽ�ӿ̶��ߣ��縩�ӿ̶��ߣ�����Ũ��ƫ�ߣ�d.Ҫ��Ũ�������ձ��ڵ���ʢˮ���ձ��У����ܽ�Ũ����ֱ�ӵ����ձ��������ձ���ע������ˮ��ϡ��Ũ���e.����ʱ��������ˮ�����̶��ߣ����ý�ͷ�ι���������������������ҺŨ��ƫ�ͣ�f.����ҡ�Ⱥ���Һ����ڿ̶��ߣ����ý�ͷ�ιܼ�����ˮ����ʹ������ҺŨ��ƫ�ͣ��������ƹ����У����в���������� abcdef����ʹ������ҺŨ��ƫ�ߵ��� abc������ţ���������ƿ��ת����Һʱ������Һ����������ƿ���潫����������ҺŨ��ƫ�ͣ�Ӧϴ������ƿ���������ơ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������(Na2S2O3)��һ�ֳ����ĵζ�������Ҫ����ԭ�ϡ�ʵ�����Ʊ���������Ƶ��������£�

�ش��������⣺
��1�����ܽ⡱ʹ�õ�ˮ���������һ��ʱ�䣬Ȼ����ȴ�����´��á���Ŀ����____________��
��2������Ӧ���еĻ�ѧ����ʽΪ_____________________________________________��
��3�������ͼ��ʾװ���Ʊ���������ƾ��壺

��Bƿ��������___________________��
��ʵ�����ȴ�________���A����C��������Һ©���Ļ�����.
��װ��D���ڴ���β������ѡ�õ������װ��Ϊ______________�����ţ���

��4��Ϊ��̽���ֲ�Ʒ�е����ʣ��������¼��貢����ʵ�飺
�ٸôֲ�Ʒ�п��ܺ��е�������Na2CO3��NaHCO3��Na2SO3��NaHSO3��S��Na2S��_____________
�����е�һ�ֻ��֡�
��ȡ������Ʒ���Թܣ���������ϡ���ᣬ������ͨ��CuSO4��Һ�У�δ������ɫ�������ݴˣ�______(��ܡ������ܡ�)��Ϊ�ֲ�Ʒ��һ��û��Na2S�������� _____________________________��
����ѡ�������Լ����ʵ�鷽���������Ʒ�к���Na2CO3��NaHCO3��
��ѡ�Լ���3 molL-1H2SO4��Һ��1 mol L-1 NaOH��Һ������KMnO4��Һ������NaHCO3��Һ��Ʒ����Һ������ʯ��ˮ��
ʵ�鷽�� | _______. |
ʵ������ | _______. |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
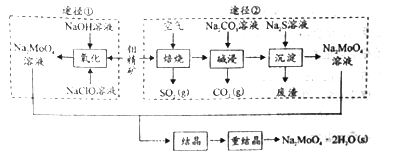
����Ŀ���о����������ƿɼ��������ĸ�ʴ���ʡ���ҵ�������⾫��(��Ҫ�ɷ��Dz�����ˮ��MoS2���������ؽ���������)�Ʊ������ƾ���( Na2MoO4��2H2O)��;����ͼ��ʾ��
�ش��������⣺
��.(1)���б�־�У�Ӧ���ڱ���NaClO�����ϵ���____________��

(2)Ϊ����߱���Ч�ʣ����������������Բ��õĴ�ʩ��__________________��
(3);�����Ʊ���������Һ��ͬʱ��SO42-���ɣ��÷�Ӧ�����ӷ���ʽΪ_____________��
(4)�ؽᾧ�õ���ĸҺ�������´��ؽᾧʱ�ظ�ʹ�ã����ﵽһ����������뾻��������ԭ����________________________��
(5)���ᾧ��ǰ��ò�������Ũ��c(MoO42-)=0.40mol/L��c(SO42-)=0.04mol/L�������Ba(OH)2�����ȥSO42-����BaMoO4��ʼ����ʱ��SO42-��ȥ����Ϊ______%��(������λ��Ч����)��
��(1)̼��������������и�ʴ���������Ũ�ȱ仯�����Բ��죬��ԭ�������_____��
(2)�����ͼ������ƣ��¹�����������Ũ��Ϊ300mg��L��1����ʴЧ�����ʱ�����Ƶ����ʵ���Ũ��Ϊ_________��������λ��Ч���֣���[��֪��Ksp(BaSO4)=1.1��10-10��Ksp(BaMoO4)=4.0��10-8������ Ba(OH)2����������Һ����仯�ɺ���]��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������һ�����ʵ���Ũ�ȵ�NaOH��Һʱ�����ʵ��������ƫ�ߵ�����������
A. ת��ʱ����Һ�������� B. ����ʱ�۲�Һ�温��
C. ������NaOH��Һ�������ձ��� D. ����ƿ��ԭ������������ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����
��������ˮ������һ��ʱ��pH��С
��Al��Fe�������������ܵ�����Ĥ�����ڲ����������ʴ
��Al2O3��Fe2O3��Ϊ������������Զ�����������
��pH��5.6��7.0֮��Ľ�ˮͨ����Ϊ����
A. �٢� B. �ڢ� C. �٢� D. �ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Na����Cl2��ȼ������(����)
A. �÷�Ӧ���ڷ��ȷ�Ӧ B. �÷�Ӧ�������ȷ�Ӧ
C. �ɼ��������ͷ����� D. �ϼ��������ͷ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��[2017����]2016��IUPAC����117��Ԫ��ΪTs����������![]() ����ti��n����Ts��ԭ�Ӻ���������������7������˵������ȷ����
����ti��n����Ts��ԭ�Ӻ���������������7������˵������ȷ����
A��Ts�ǵ������ڵ���A��Ԫ�� B��Ts��ͬλ��ԭ�Ӿ�����ͬ�ĵ�����
C��Ts��ͬ��Ԫ���зǽ��������� D��������Ϊ176��Ts���ط�����![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������BN����һ����Ҫ�Ĺ����մɲ�������Ȼ��ɰΪ��ʼ�����һϵ�з�Ӧ���Եõ�BF3��BN������ͼ��ʾ��

��ش��������⣺
��1��д��B2O3��NH3��Ӧ����BN�ķ���ʽ___________________________
��2����̬Nԭ�ӵļ۲�����Ų�ʽΪ_________��
��3��B��N��O��ȣ���һ������������_______��BN��BԪ�صĻ��ϼ�Ϊ_____
��4��SO42�C��Sԭ�ӵ��ӻ��������Ϊ_______��O��S��O�ļ�����_______��д��һ����SO42�C�ȵ��������_______��
��5��BF3����NH3��Ӧ����BF3NH3��BF3���ӵķ��ӹ���Ϊ_______��BF3NH3��BF3��NH3֮��ͨ��___________������Ӽ������� ��λ������������� ��ϡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A(C2H4)�ǻ������л�����ԭ�ϡ���A�ͳ������л���ɺϳ�һ���������Ϻ�һ����ȩ�����ϣ�����ϳ�·����ͼ��ʾ�����ַ�Ӧ������ȥ����

��֪��

�ش��������⣺
��1��B�ķ���ʽ��___________��C�к��еĹ����������� ____________��
��2����DΪ��ȡ�������廯��������������Ʒ�Ӧ��ÿ��D������ֻ����1����ԭ�ӣ�D����Ԫ�ص���������ԼΪ13.1%����D�Ľṹ��ʽΪ___________���ķ�Ӧ������________________��
��3���ݱ�������Ӧ�����������£���NaHSO4��H2OΪ�������У���д���˷�Ӧ�Ļ�ѧ����ʽ��____________________________________��
��4����д���������������ı���ȩ������ͬ���칹��Ľṹ��ʽ��___________________��
i .���б�����![]() �ṹ
�ṹ
ii.�˴Ź���������4��壬�ҷ����֮��Ϊ3��2��2��1
��5����������EΪ�����ѵ�ͬϵ�����Է��������ȱ����Ѵ�14������ʹFeCl3��Һ��ɫ��E������ͬ���칹�干��(�����������칹)________________�֡�
��6������ �ĺϳ�·�ߣ�д����2-�ȱ���ͱ�Ҫ�����Լ��Ʊ�
�ĺϳ�·�ߣ�д����2-�ȱ���ͱ�Ҫ�����Լ��Ʊ� �ĺϳ�����ͼ��_______________________________________
�ĺϳ�����ͼ��_______________________________________
�ϳ�����ͼʾ�����£�CH2 = CH2![]() CH3CH2Br
CH3CH2Br![]() CH3CH2OH
CH3CH2OH
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com