
����Ŀ��ԭ���������������A��B��C��D��E��F����Ԫ�ء�����A�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��еĵ�������ȣ�C�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Aԭ�ӵ���ͬ��DΪ������������ԭ�Ӱ뾶��������Ԫ�أ�E��Cλ��ͬһ���壬F��ԭ������Ϊ29��
(1)Fԭ�ӻ�̬�ĺ�������Ų�ʽΪ_____________________��
(2)��A��B��C����Ԫ���У���һ��������С�����˳����________(��Ԫ�ط��Żش�)��
(3)Ԫ��B�ļ���̬�⻯��ķе�Զ����Ԫ��A�ļ���̬�⻯��ķе㣬����Ҫԭ����______________________________��
(4)��A��B��C�γɵ�����CAB����AC2��Ϊ�ȵ����壬��CAB���ĽṹʽΪ___________________________��
(5)��Ԫ��A��E���γɵij����������У�Aԭ�ӹ�����ӻ�����Ϊ________��
(6)��B��C��D����Ԫ���γɵĻ����ᄃ��ľ�����ͼ��ʾ����û�����Ļ�ѧʽΪ_______________________________��
���𰸡�(1)[Ar]3d104s1 (2)C<O<N (3)NH3���Ӽ�������(4)[NCO]�� (5)sp (6)NaNO2
��������A�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��еĵ�������ȣ�AΪCԪ�أ�����C�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Aԭ�ӵ���ͬ��CΪOԪ�أ���BΪNԪ�أ�����E��Cλ��ͬһ���壬EΪSԪ�أ�DΪ������������ԭ�Ӱ뾶��������Ԫ�أ���ԭ������С��E��DΪNaԪ�أ�F��ԭ������Ϊ29��FΪCuԪ�ء�(1)Cu�ĺ�������Ų��Ƚ����⣬�۵����Ų�Ϊ3d104s1��(2)����Nԭ�ӵ�2p������ڰ��������һ������N>O������C��N��O�ĵ�һ��������С�����˳����C<O<N��(3)NH3���Ӽ��������CH4����֮��û����������Էе�NH3Զ����CH4��(4)OCN����CO2��Ϊ�ȵ����壬��ۼ��ṹ���ƣ���ṹʽΪ[NCO]����(5)C��S�γɵij�����������CS2��Cԭ�ӹ�����ӻ�����Ϊsp��(6)N��O��Na����Ԫ���γɵĻ�����ľ����У������ӦΪNa���������ϵ���ԭ������ӦΪNO![]() ���û�����Ļ�ѧʽΪNaNO2��
���û�����Ļ�ѧʽΪNaNO2��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����3.20g Cu��50.0mL l0.0mol/L��HNO3��ַ�Ӧ����ԭ������NO��NO2������Ӧ����Һ����xmolH+�����ʱ��Һ�к�NO3-�����ʵ���Ϊ( )
A. xmol B. (x+0.1) mol C. 2xmol D. 0.1xmol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA���������ӵ���������ֵ��������˵����ȷ����( )
A��1.8 g D2O����NA������
B����5 mL 3 mol/L FeCl3��Һ�Ƴɵ�������������������������Ϊ0.015NA
C����Na2O2��CO2�ķ�Ӧ�У�ÿת��NA������ʱ������22.4 L��CO2
D��25 ��ʱ��7 g C2H4��C3H6�Ļ�������У�����NA��C��H��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Fe2����Fe3����O![]() ��CN����F���л����ӵ��γɵĻ�������й㷺��Ӧ�á�
��CN����F���л����ӵ��γɵĻ�������й㷺��Ӧ�á�
(1)C��N��Oԭ�ӵĵ�һ�������ɴ�С��˳����___________________________��
(2)Fe2����̬��������Ų�ʽΪ__________________________��
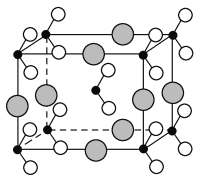
(3)��������ï���dz������Ϳ��������ṹ��ͼ1��ʾ��
��������̼ԭ�ӵ��ӻ���ʽ��______________________��
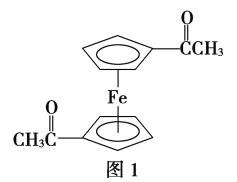
(4)�����K3Fe(CN)6�����ڵ��Ӵ������������������廥Ϊ�ȵ������һ�ַ��ӵĻ�ѧʽΪ________________����֪(CN)2��ֱ���ͷ��ӣ������жԳ��ԣ���(CN)2�Цм��ͦҼ��ĸ�����Ϊ___________________________��
(5)F����������Fe3���γ�[FeF6]3������������Mg2����K���γ�һ��������ϵ�����Ӿ��壬�˾���Ӧ���ڼ������ṹ��ͼ2��ʾ��
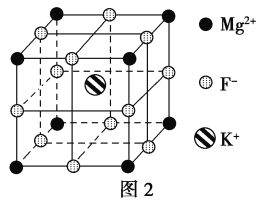
�þ���Ļ�ѧʽΪ_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��[2017����]�����й�������������;���ж�Ӧ��ϵ����
A��Na2O2����CO2����O2��������������߹�����
B��ClO2���л�ԭ�ԣ�����������ˮ��ɱ������
C��SiO2Ӳ�ȴ�����������ά
D��NH3������ˮ�������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25��ʱ����������0.1mol/L��HCl��Һ��0.06mol/L��Ba(OH)2��Һ����Һ��pHֵ���ڣ� ��
A��2.0 B��12.3 C��1.7 D��12.0
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��Һ�п��ܺ���Cl-��SO42-��CO32-��NH4+��Fe3+��Fe2+��Al3+��Na+��ijͬѧΪ��ȷ����ɷ֣�ȡ������Һ����Ʋ����������ʵ�飺
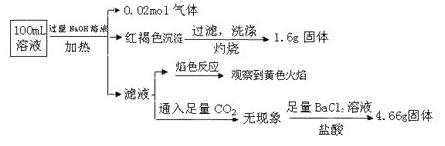
����˵����ȷ���ǣ� ��
A. ԭ��Һ��c(Fe3+)=0.2 molL-1
B. ��Һ��������4�����Ӵ��ڣ�����Cl-һ�����ڣ���c(Cl-)��0.2 mol��L-1
C. SO42����NH4+��Na+һ�����ڣ�CO32��һ��������
D. Ҫȷ��ԭ��Һ���Ƿ���Fe2+�������Ϊ��ȡ����ԭ��Һ���Թ��У�����������ˮ���������ټ�KSCN��Һ����Һ��Ѫ��ɫ������Fe2+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��T��ʱ���мס��������ܱ������������������Ϊ1L�������������Ϊ2L���ֱ���ס����������м���6molA��3molB��������Ӧ���£�3A��g����bB��g��![]() 3C��g����2D��g����H<0;4minʱ�������ڵķ�Ӧǡ�ôﵽƽ�⣬
3C��g����2D��g����H<0;4minʱ�������ڵķ�Ӧǡ�ôﵽƽ�⣬
A��Ũ��Ϊ2.4mol/L��B��Ũ��Ϊ1.8mol/L;tminʱ�������ڵķ�Ӧ��ƽ�⣬
B��Ũ��Ϊ0.8mol/L.���������Ϣ�ش��������⣺
��1���������з�Ӧ��ƽ������v��B����______________��
��2���������з�Ӧ�ﵽƽ��ʱ����ʱ��t__________4 min������ڡ�����С�ڡ����ڡ�����
��3����Ҫʹ�ס���������B��ƽ��Ũ����ȣ����Բ�ȡ�Ĵ�ʩ��______________��
A�������¶Ȳ��䣬����������������2 L
B����������������䣬ʹ�����������¶�
C����������������¶ȶ����䣬����м���һ������A����
D����������������¶ȶ����䣬����м���һ������B����
��4�����¶��£������Ϊ1L���ܱ�������ͨ��A��B��C��D�������ʵ����ֱ�Ϊ3mol��1mol��3mol��2mol����ʱ��Ӧ________________������ڻ�ѧƽ��״̬������������Ӧ������С������淴Ӧ������С�����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com