
����Ŀ��I. ij�л�����C��H��O����Ԫ����ɣ����ģ����ͼ��ʾ��

��1�����л���ķ���ʽ��_____________��
��2�����л�����Է����Ӿ۷�Ӧ�������Ľṹ��ʽ��_____________��
��3�������йظ��л���������У���ȷ����____������ţ���
a. ����NaHCO3��Һ��Ӧ
b. �ܷ���ˮ�ⷴӦ
c. ���������CCl4��Һ��Ӧ
d. �������Ը��������Һ��Ӧ
II. ��1��д�����л���������ƻ�ṹ��ʽ��
��![]() _______________________________��
_______________________________��
��2,5-����-2,4-����ϩ�Ľṹ��ʽ��_______________________��
��2��������ֳƻƼ���ҹ��ض�����ҩ������������е�һ���������ҹ���ѧ���о�������ṹ��ͼ��

������к��������ŵ�������_________________������____________�ࣨ������ӡ�����
���𰸡� C3H4O2 ![]() ad ��������
ad �������� ![]() �ǻ� ��
�ǻ� ��
��������I. �����л�������ģ�Ϳ�֪���л���ṹ��ʽΪCH2=CHCOOH����
��1�����л���ķ���ʽ��C3H4O2����2�����л��ﺬ��̼̼˫�������Է����Ӿ۷�Ӧ�������Ľṹ��ʽ��![]() ����3�������йظ��л���������У���ȷ����____������ţ���
����3�������йظ��л���������У���ȷ����____������ţ���
a. �����Ȼ�������NaHCO3��Һ��Ӧ��a��ȷ��b. ���������������ܷ���ˮ�ⷴӦ��b����c. ����̼̼˫�����������CCl4��Һ��Ӧ��c����d. ����̼̼˫�����������Ը��������Һ��Ӧ��d��ȷ����ѡad��
II. ��1����![]() �����࣬�����Ǽ�����������2��5-����-2��4-����ϩ�Ľṹ��ʽΪ
�����࣬�����Ǽ�����������2��5-����-2��4-����ϩ�Ľṹ��ʽΪ![]() ����2����������صĽṹ��ʽ��֪�����к��������ŵ��������ǻ������ڴ���
����2����������صĽṹ��ʽ��֪�����к��������ŵ��������ǻ������ڴ���


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������һ����Ҫ�Ļ�����Ʒ���ǵ��ʹ�ҵ���л��ϳɹ�ҵ�Լ��������ᡢ��κʹ���ȵ�ԭ�ϡ�
��1����һ���¶��£��ڹ̶�������ܱ������н��п��淴Ӧ��N2+3H2![]() 2NH3���ÿ��淴Ӧ�ﵽƽ��ı�־��________________��
2NH3���ÿ��淴Ӧ�ﵽƽ��ı�־��________________��
A��3v(H2)��=2v(NH3)��
B����λʱ������m mol N2��ͬʱ����3m mol H2
C�������ڵ���ѹǿ������ʱ����仯
D�����������ܶȲ�����ʱ��仯
E��a molN��N�����ѵ�ͬʱ����6amolN��H������
F��N2��H2��NH3�ķ�����֮��Ϊ1��3��2
��2��ij��ѧ�о���ѧϰС��ģ�ҵ�ϳɰ��ķ�Ӧ�����ݻ��̶�Ϊ2L���ܱ������ڳ���1molN2��3molH2��������ʴ�����������Ժ��Բ��ƣ�����һ���¶�ѹǿ�¿�ʼ��Ӧ������ѹ���Ƽ��������ѹǿ�ı仯���£�
��Ӧʱ��/min | 0 | 5 | 10 | 15 | 20 | 25 | 30 |
ѹǿ/MPa | 16.80 | 14.78 | 13.86 | 13.27 | 12.85 | 12.60 | 12.60 |
��ӷ�Ӧ��ʼ��25minʱ����N2��ʾ��ƽ����Ӧ����= �����¶���ƽ�ⳣ��K= ��
��3�� ���úϳ�������Ҫ�ɷ�ΪCO��CO2��H2���ڴ����������ºϳɼ״�����Ҫ��Ӧ���£�
��CO(g)+2H2(g)![]() CH3OH(g) ��H=��99kJ��mol��1
CH3OH(g) ��H=��99kJ��mol��1
��CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ��H=��58 kJ��mol��1
CH3OH(g)+H2O(g) ��H=��58 kJ��mol��1
��CO2(g)+H2(g)![]() CO(g)+H2O(g) ��H
CO(g)+H2O(g) ��H
ͼ1������ȷ��ӳƽ�ⳣ��K���¶ȱ仯��ϵ�������� ����Ӧ������H= kJ��mol��1��
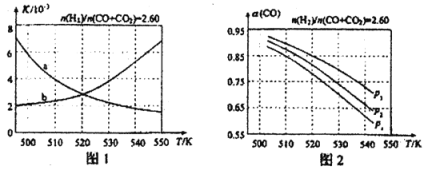
��4���ϳ��������n(2)/n(CO+CO2)=2.60ʱ��ϵ�е�COƽ��ת������(CO)���¶Ⱥ�ѹǿ�Ĺ�ϵ��ͼ2��ʾ��ͼ�е�ѹǿp1��p2��p3�ɴ�С��˳��Ϊ ����(CO)���¶����߶���С����ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й��ڵ���ƽ�ⳣ����K����˵������ȷ����
A. ��һ���¶ȣ���ͬŨ��ʱ����ƽ�ⳣ����K��Խ����Ա�ʾ������ʵ���̶�Խ��
B. ����ƽ�ⳣ����K�����¶���
C. ��ͬŨ�ȵ�ͬһ������ʣ������ƽ�ⳣ����K����ͬ
D. ��Ԫ�����������ƽ�ⳣ�����ϵΪK1<K2<K3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij���ӷ���������Ҫ��Pt��Al2O3��ʯī�����л��պ��ಬ�����������塣�����������£�
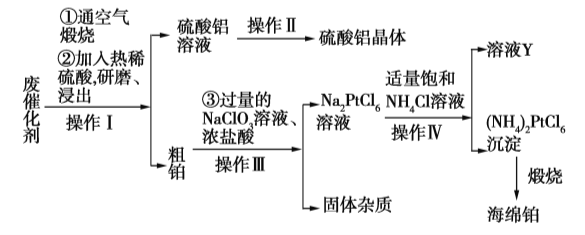
��ش��������⣺
��1��������ͨ��������յ�Ŀ����_____________________________��
��2����������������ĥ����������ֱ�ӽ�����ԭ����_____________________________��
��3��д����������Ӧ�����ӷ���ʽ��_____________________________��
��4���������е��ĸ�����������������ͬ�ģ���������__________����ʵ���ҽ��д˲���ʹ�õ���Ҫ����������___________________����ҺY�п���ѭ��ʹ�õ�������____________���ѧʽ����
��5�������ζ����ⶨ���������������ĺ�������ȡ����������0.5400 g����2.0 mL 3.0 mol��L1�������ܽ���Ƴ�250 mL��Һ��ȡ25.00 mL����ƿ�У�����0.02 mol��L1��EDTA��Һ20.00 mL��������Һ������5 min����ȴ�����£���2�ζ��ӳ�ָʾ������0.02 mol��L1�ı�ZnSO4��Һ�ζ����յ�����5.00 mL����þ�����������������Ϊ___________������֪Al3+��Zn2+��EDTA��Ӧ�ķ���ʽ���£�Al3++H2Y2![]() AlY+2H+��H2Y2+Zn2+
AlY+2H+��H2Y2+Zn2+![]() ZnY2+2H+��
ZnY2+2H+��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij������ҩ��ĺϳ��м���ĺϳ�·��������

��ش��������⣺
��1��X�����еĹ���������Ϊ____________��Y���ӵĽṹ��ʽΪ_______________��![]() ��һ��ͬ���칹��ΪCH3C��CCH3��������Ϊ__________________��
��һ��ͬ���칹��ΪCH3C��CCH3��������Ϊ__________________��
��2���ںϳ�·�����������ķ�Ӧ���ͷֱ�Ϊ��_____________����______________��
��3��д����Ӧ�������Ļ�ѧ����ʽ����Ӧ��____________________________________��
��Ӧ��____________________________________��
��4��![]() ���е�������__________________������ţ���
���е�������__________________������ţ���
���ܷ���ȡ����Ӧ�����ܷ����ӳɷ�Ӧ�����ܷ����кͷ�Ӧ��
���ܷ���������Ӧ�����ܷ�����ȥ��Ӧ��
��5���ÿ�����ҩ��ĺϳ��м����ж���ͬ���칹�壬���������������Ľṹ��ʽΪ__________________��
���ɷ���������Ӧ��������NaHCO3��Ӧ����CO2�����Ƿ����廯���
���˴Ź���������ʾ��4�ֲ�ͬ��ѧ��������ԭ�ӡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ�����ӵ�������������������ȷ����
A. ������64 g S4��S8������к���ԭ����Ϊ2NA
B. ��״���£�22.4LHF�к�������Ϊ10NA
C. ��״��ʱ��2.24LNO��1.12LO2���ܱ������г�ַ�Ӧ���������Ϊ0.1NA
D. ��������1mol�����м��ȷ�Ӧ����ʧȥ�ĵ�����Ϊ3NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������A~F��������Ϥ�ĵ��ʻ������A��B���dz����Ľ�������B�ĺϽ��������E�ڳ������ǻ���ɫ���壻���ʵ��������£�����֮����Է�������ͼ��ʾ��ת����

�Իش��������⣺
�� A�Ļ�ѧʽ��____________��
�� ��ӦF��D�Ļ�ѧ����ʽ��______________________________________��
�� ����F�н��������ӵij��÷�����___________________________________��
�� ����B�����ᷴӦ�����ӷ���ʽ��_________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ھ������е����Ų�ʽ��ԭ���У�ԭ�Ӱ뾶�����ǣ� ��
A. 1s22s22p63s23p1 B. 1s22s22p1 C. 1s22s22p3 D. 1s22s22p63s23p4
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com