
����Ŀ���Ȼ���(HSO3Cl)���Ǿ����ǰ�ҩ������������Ҫ��Ӧ�á����³�ѹ���Ȼ���Ϊ��ɫ��״Һ�壬�е�ԼΪ152�棬��ʪ�Ժ�ʴ�Լ�ǿ���ڿ����з��̡�ѧϰС����ʵ������SO3��HCl���Ʊ�HSO3Cl���ⶨ��Ʒ���ȡ��������ʵ��(�г�װ����ȥ)����ش��������⣺
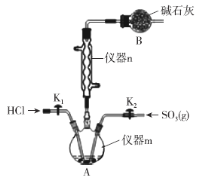
��1������m������Ϊ___��
��2����֪��HSO3Cl����Ԫ��Ϊ��6�ۣ�Oԭ�Ӻ�Clԭ�ӵ�����������8�����ȶ��ṹ����HSO3Cl�еĻ�ѧ��Ϊ___ (�������Ӽ����������Լ��������Ǽ��Լ���)��
��3��SO3����������������Ũ���Ṳ���Ʊ���������Ӧ�Ļ�ѧ����ʽΪ___��
��4��װ��B������Ϊ___��������n��֪�Ʊ�HSO3Cl�ķ�ӦΪ___ (�������ȷ�Ӧ���������ȷ�Ӧ��)��
��5��HSO3Cl���ȵIJⶨ(����m�еõ���HSO3Cl�г�����������SO3)��
i.ȡ25.0g��Ʒ����ˮ�У����������Ba(NO3)2��Һ��ַ�Ӧ���ˡ�
ii.����Һ�м��������AgNO3��Һ�����ˡ�ϴ�ӡ�����������������ó���AgCl������Ϊ28.7g��
��HSO3Cl��ˮ������Ӧ�Ļ�ѧ����ʽΪ___��
�ڲ�ƷHSO3Cl�Ĵ���Ϊ___��
���𰸡�������ƿ ���Լ� P2O5��3H2SO4��Ũ��![]() 3SO3����2H3PO4 ��ֹ�����е�ˮ������ �뷴Ӧװ��A�У������ջӷ�������HCl���� ���ȷ�Ӧ HSO3Cl+H2O=HCl+H2SO4 93.2%
3SO3����2H3PO4 ��ֹ�����е�ˮ������ �뷴Ӧװ��A�У������ջӷ�������HCl���� ���ȷ�Ӧ HSO3Cl+H2O=HCl+H2SO4 93.2%
��������
��1������m������Ϊ������ƿ���ʴ�Ϊ��������ƿ��
��2��HSO3Cl����Ԫ��Ϊ��6�ۣ�Oԭ�Ӻ�Clԭ�ӵ�����������8�����ȶ��ṹ���ṹʽΪ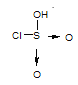 ����HSO3Cl�еĻ�ѧ��Ϊ���Լ� ���ʴ�Ϊ�����Լ���
����HSO3Cl�еĻ�ѧ��Ϊ���Լ� ���ʴ�Ϊ�����Լ���
��3��SO3����������������Ũ���Ṳ���Ʊ���������Ӧ�Ļ�ѧ����ʽΪP2O5��3H2SO4��Ũ��![]() 3SO3����2H3PO4���ʴ�Ϊ��P2O5��3H2SO4��Ũ��
3SO3����2H3PO4���ʴ�Ϊ��P2O5��3H2SO4��Ũ��![]() 3SO3����2H3PO4��
3SO3����2H3PO4��
��4��HSO3Cl��ˮ����ҷ�Ӧ��������m���ݳ���HCl��Ⱦ������װ��B������Ϊ��ֹ�����е�ˮ�������뷴Ӧװ��A�У������ջӷ�������HCl���塣����n����������������HSO3Cl����֪�Ʊ�HSO3Cl�ķ�ӦΪ���ȷ�Ӧ���ʴ�Ϊ����ֹ�����е�ˮ�������뷴Ӧװ��A�У������ջӷ�������HCl���壻���ȷ�Ӧ��
��5����HSO3Cl��ˮ������Ӧ�Ļ�ѧ����ʽΪHSO3Cl+H2O=HCl+H2SO4��
�ڸ���������Ϣ��HSO3Cl~AgCl������HSO3Cl�Ĵ���Ϊ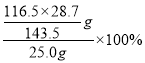 =93.2%��
=93.2%��
�ʴ�Ϊ��HSO3Cl+H2O=HCl+H2SO4��93.2%��


 ��ʦ�㲦��ϵ�д�
��ʦ�㲦��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������Ԫ��X��Y��Z��W��ԭ������������������ԭ�ӵ�����������֮��Ϊ14��Z�Ƕ���������Ԫ����ԭ�Ӱ뾶����Ԫ�أ�Z��Xԭ�ӵ�������������ͬ��Y��Wͬ���塣����˵����ȷ���ǣ� ��
A.X��Yֻ���γ�һ�ֻ�����
B.ԭ�Ӱ뾶��r(Y)��r(W)��r(Z)
C.W�ļ���̬�⻯������ȶ��Ա�Y��ǿ
D.Z������������Ӧ��ˮ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��SO2�㷺����ҽҩ�����Ṥҵ�������շ����е�SO2�������·�����
������ | �ü�ʽ������Al2(SO4)x(OH)y��Һ���ո���SO2 |
������ | ��Fe2+��Fe3+���£��ÿ���(O2)��SO2����ΪH2SO4 |
(1)������Ĺ������¡�
�� �Ʊ�Al2(SO4)x(OH)y����Al2(SO4)3��Һ�м���CaO��ĩ����pH��3.6�� CaO��������______
�� ���գ�Al2(SO4)x(OH)y����SO2��IJ�����______(д��ѧʽ)��
�� ���������Ȣ��в������SO2��Al2(SO4)x(OH)y������
(2)�������У���Fe2+���£�SO2��O2��H2O����H2SO4�Ļ�ѧ����ʽ��______��
(3)�������У�Fe2+�Ĵ����̿ɱ�ʾ���£�
����2Fe2++O2+SO2=2Fe3++SO42-
��������
�� д���������ӷ���ʽ��______��
�� ����ʵ�鷽����֤ʵ���������̡���ʵ�鷽������������
a.��FeCl2��Һ����KSCN���ޱ仯
b.��FeCl2��Һͨ������SO2������KSCN����ɫ��졣
c.ȡb����Һ��______��
(4)�������У����������õζ����ⶨ�����в���SO2�ĺ�������V L(�ѻ���Ϊ��״��)�����е�SO2��1%��H2O2��ȫ���գ�����Һ����ͼ��ʾװ�õζ���������a mL c mol/L NaOH��Һ��

��H2O2����SO2�Ļ�ѧ����ʽ______��
�� �����в���SO2���������Ϊ______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��W��Ϊ������Ԫ�أ�������Ԫ�����ڱ��е�λ����ͼ��ʾ����Yԭ�ӵ������������Ǵ�����������3��������˵������ȷ����( )

A.ԭ�Ӱ뾶��W��Z��Y��X
B.����������Ӧˮ��������ԣ�Z��W��X
C.����Ԫ�صĵ����У�Z���ʵ��ۡ��е����
D.W�ĵ�������ˮ��Ӧ������һ�־���Ư���Ե�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)�һ��嶡����(��ETBE��ʾ)��һ�����������ĸ�����ֵ���͵��ͼ������Ҵ����춡ϩ(��IB��ʾ)�ڴ���HZSM��5���ºϳ�ETBE����Ӧ�Ļ�ѧ����ʽΪ��C2H5OH(g)+IB(g)=ETBE(g) ��H���ش��������⣺
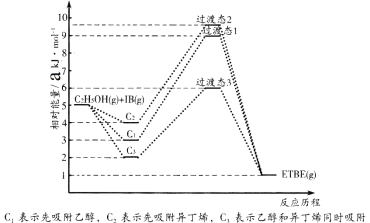
��Ӧ�ﱻ����HZSM��5������˳���뷴Ӧ���̵Ĺ�ϵ��ͼ��ʾ���÷�Ӧ�ġ�H=__________ kJ��mol-1����Ӧ���̵�����;����________(��C1��C2��C3)��
(2)���������Դ�ǵ����о���һ���ȵ����⡣������(CH3OCH3)��δ������������ͺ�Һ������Ϊ�ྻҺ��ȼ��ʹ�ã���ҵ����CO��H2Ϊԭ������CH3OCH3����ҵ�Ʊ��������ڴ���Ӧ����(ѹ��2.0��10.0Mpa���¶�230��280��)�������з�Ӧ��
��Ӧ����CO(g)+2H2(g)CH3OH(g) ��H1=-99kJ��mol1
��Ӧ����2CH3OH(g)CH3OCH3(g)+H2O(g) ��H2=-23.5kJ��mol1
��Ӧ����CO(g)+H2O(g)CO2(g)+H2(g) ��H3=-41.2kJ��mol1
���ڸ������£�����Ӧ1����ʼŨ�ȷֱ�Ϊ��c(CO)=0.6mol��L1��c(H2)=1.4mol��L1��8min��ﵽƽ�⣬CO��ת����Ϊ50%����8min��H2��ƽ����Ӧ����Ϊ__________��
����t��ʱ����Ӧ2��ƽ�ⳣ��Ϊ400�����¶��£���1L���ܱ������м���һ���ļ״�����Ӧ��ijʱ�̲�ø���ֵ����ʵ���Ũ�����£�
���� | CH3OH | CH3OCH3 | H2O |
c(mol��L1) | 0.46 | 1.0 | 1.0 |
��ʱ��v��___v��(���������������)��ƽ��ʱc(CH3OCH3)�����ʵ���Ũ����___��
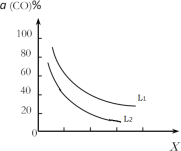
�۴���Ӧ�ҵ��ܷ�Ӧ3CO(g)+3H2(g)CH3OCH3(g)+CO2(g)��CO��ƽ��ת������(CO)���¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��ͼ��X����___(��¶ȡ���ѹǿ��)����L1___L2(���������������)��
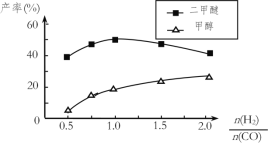
���ڴ�����������ͬʱ����������Ӧ������������ʼͶ�ϱ�![]() �ĸı䣬�����Ѻͼ״��IJ���(�����е�̼ԭ��ռ��ʼCO��̼ԭ�ӵİٷ���)������ͼ�ı仯���ơ��Խ���Ͷ�ϱȴ���1.0֮������Ѳ��ʺͼ״����ʱ仯��ԭ��______________��
�ĸı䣬�����Ѻͼ״��IJ���(�����е�̼ԭ��ռ��ʼCO��̼ԭ�ӵİٷ���)������ͼ�ı仯���ơ��Խ���Ͷ�ϱȴ���1.0֮������Ѳ��ʺͼ״����ʱ仯��ԭ��______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͭ�Ķ��ֻ����������������ж��й㷺��;����ش��������⣺
��1��Cu2O��CuO��ͭ������������ɻ���ת������֪��
i.2Cu2O(s)��O2(g)=4CuO(s) ��H=-292.0kJ��mol-1
ii.C(s)��2CuO(s)=Cu2O(s)��CO(g) ��H=��35.5kJ��mol-1
��CO��ȼ����Ϊ283.0kJ��mol-1����C(s)��ȼ����Ϊ___��
��2��Cu2O��CuO������������
�����ӽ���Ĥȼ�ϵ��(PEMFC)��ȼ�����г�����H2�����������CO��CO2������CO��PEMFC���������ض�����������CuO/CeO2���������������ѳ�CO��160�桢��CuO/CeO2������ʱ������CO�Ļ�ѧ����ʽΪ___���ֱ���HIO3��H3PO4��CuO/CeO2���д�������һ�������£����ò�ͬ��������CO�����ĶԱ�ʵ�飬����ͼ���ߣ����д���___ (����b������c��)��������ã�120��ʹ�ô���b������������ȼ������CO���������Ϊ0.71%����������Ϊ2000mL��h-1����1h��CO���Ϊ___mL��

����Cu2O�������ºϳ�CH3OH����Ӧ���£�CO(g)��2H2(g)![]() CH3OH(g) ��H=-90.0kJ��mol-1����������߸÷�ӦCO��ƽ��ת���ʵ�������___(����)��
CH3OH(g) ��H=-90.0kJ��mol-1����������߸÷�ӦCO��ƽ��ת���ʵ�������___(����)��
A.���µ�ѹ B.���¸�ѹ C.���¸�ѹ D.���µ�ѹ
T��ʱ����CO��H2��һ��������Ϻ�Ͷ���ݻ�Ϊ2L�ĺ����ܱ������У�CO����ʼŨ��Ϊ1.0mol��L-1��ƽ��ʱ�������ϵ�У�n(H2)=1.4mol��n(CH3OH)=1.7mol����Ӧ�ﵽƽ��ʱCO��ת����Ϊ___������Ӧ�ﵽƽ��״̬���������������䣬�ٳ���0.2molCO��0.2molCH3OH��ƽ����___(����������������)��Ӧ�����ƶ���������___��
��3��CuS�ʺ�ɫ���������ܵ�����֮һ����������������ʹ��һЩ���Ʋ����ܵķ�Ӧ���Է�������0.01mol��L-1CuSO4��Һ�У�����ͨ��H2Sά�ֱ���(H2S����Ũ��Ϊ0.1mol��L-1)��������Ӧ��H2S(aq)��Cu2��(aq)![]() CuS(s)��2H��(aq)���÷�Ӧ�Ļ�ѧƽ�ⳣ��KΪ___(����2λ��Ч����)����֪��Ka1(H2S)=1.1��10-7��Ka2(H2S)=1.3��10-13��Ksp(CuS)=6.3��10-36��
CuS(s)��2H��(aq)���÷�Ӧ�Ļ�ѧƽ�ⳣ��KΪ___(����2λ��Ч����)����֪��Ka1(H2S)=1.1��10-7��Ka2(H2S)=1.3��10-13��Ksp(CuS)=6.3��10-36��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��Һ��ֻ���ܺ���K����NH4+��Fe2����Al3����Cl����SO42-��CO32-��AlO2���е����������ӣ�����Ũ�Ⱦ�Ϊ0.1mol��L��1��ijͬѧ����������ʵ�飬����˵����ȷ����

A. ��ȷ��ԭ��Һ���Ƿ���Al3����Cl��
B. ԭ��Һ�д���NH4+��Fe2����Cl����SO42-
C. ��ȷ������C�ijɷ�
D. ��ҺX�д������ڵ���������NH4+��Fe2����Ba2+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��þϡ���Ͻ�㷺Ӧ���ں��ա����졢���ӡ�ͨѶ����������ҵ���������ε�ⷨ��һ�ָ�Ч�Ʊ�þϡ���Ͻ�ķ�����ʵ������ȡMg-Gd�Ͻ�(���ʵ�����Ϊ1��1)�ĵ���ʾ��ͼ����(���ԭ������Mg��24��Gd��157)������˵����ȷ����

A.����LiF��BaF2�������Ǵ���
B.�����ĵ缫��ӦʽΪ2F����2e��= F2 ��
C.����·��ͨ��0.1mol����ʱ������Mg- Gd�Ͻ������Ϊ3.62 g
D.������������̼�缫��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ԫ��X��Y��Z��Ԫ�����ڱ��е�λ����ͼ��ʾ������˵����ȷ����( )
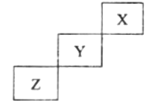
A.�ǽ�����ǿ��: Z> Y
B.��̬�⻯����ȶ���: Y>Z
C.����������ˮ��������: Y>Z
D.��ѹ��X�ĵ��ʻ�ѧ���ʷdz�����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com