
ЎѕМвДїЎїУР»ъОпKКЗДіТ©ОпµДЗ°МеЈ¬єПіЙВ·ПЯИзНјЛщКѕЈє
ТСЦЄЈєR-CN ![]()
![]() Ј»
Ј»
![]()
![]() +
+![]()
ЈЁ1Ј©BµДЅб№№јтКЅКЗ_____________ЎЈ
ЈЁ2Ј©·ґУ¦ўЫµД»ЇС§·ЅіМКЅКЗ__________________ЎЈ
ЈЁ3Ј©EКфУЪМюЈ¬Жд·ЦЧУКЅКЗ_________________ЎЈ
ЈЁ4Ј©HЦРє¬Сх№ЩДЬНЕµДГыіЖКЗ_________________ЎЈ
ЈЁ5Ј©РґіцВъЧгПВБРМхјюµД KµДТ»ЦЦН¬·ЦТм№№МеµДЅб№№јтКЅЈє________________________
ўЩУцВИ»ЇМъИЬТєПФЧПЙ«Ј» ўЪІ»є¬ГСјьЈ» ўЫєЛґЕ№ІХсЗвЖЧПФКѕ·еГж»эЦ®±ИОЄ2:12:2:2:1
ЈЁ6Ј©HѕИэІЅ·ґУ¦єПіЙKЈ¬РґіцJЎъKµД»ЇС§·ЅіМКЅ_____________________________ЎЈ
![]() .
.
Ўѕґр°ёЎїHOCH2CH2CH2OH HOOCCH2COOH + 2CH3CH2OH ![]() 2H2O + C2H5OOCCH2COOC2H5 C6H12 хҐ»щ
2H2O + C2H5OOCCH2COOC2H5 C6H12 хҐ»щ ![]()

ЎѕЅвОцЎї
ёщѕЭHЎўG·ЦОцЈ¬ФЩБЄПµEµЅGµД±д»ЇЈ¬EУ¦ёГКЗ»·јєП©Ј¬![]() Ј¬·ўЙъјУіЙЙъіЙ»·јєґјЈ¬
Ј¬·ўЙъјУіЙЙъіЙ»·јєґјЈ¬![]() Ј¬·ўЙъґЯ»ЇСх»ЇЈ¬±дОЄ»·јєНЄјґОЄ
Ј¬·ўЙъґЯ»ЇСх»ЇЈ¬±дОЄ»·јєНЄјґОЄ![]() Ј¬DОЄC2H5OOCCH2COOC2H5Ј¬
Ј¬DОЄC2H5OOCCH2COOC2H5Ј¬![]() Ј¬·ўЙъхҐ»Ї·ґУ¦Ј¬ФтCОЄHOOCCH2COOHЈ¬
Ј¬·ўЙъхҐ»Ї·ґУ¦Ј¬ФтCОЄHOOCCH2COOHЈ¬![]() У¦·ўЙъЛ®Ѕв·ґУ¦Ј¬ФтНЖіцAОЄBrCH2CH2CH2BrЈ¬BОЄHOCH2CH2CH2OHЈ¬
У¦·ўЙъЛ®Ѕв·ґУ¦Ј¬ФтНЖіцAОЄBrCH2CH2CH2BrЈ¬BОЄHOCH2CH2CH2OHЈ¬![]() ·ўЙъСх»Ї·ґУ¦Ј¬ґј±дОЄфИЛбµД·ґУ¦ЎЈ
·ўЙъСх»Ї·ґУ¦Ј¬ґј±дОЄфИЛбµД·ґУ¦ЎЈ
ўЕёщѕЭ![]() У¦·ўЙъЛ®Ѕв·ґУ¦Ј¬ФтНЖіцAОЄBrCH2CH2CH2BrЈ¬BОЄHOCH2CH2CH2OHЈ¬
У¦·ўЙъЛ®Ѕв·ґУ¦Ј¬ФтНЖіцAОЄBrCH2CH2CH2BrЈ¬BОЄHOCH2CH2CH2OHЈ¬
№Кґр°ёОЄЈєHOCH2CH2CH2OHЈ¬
ўЖ·ґУ¦ўЫµД»ЇС§·ЅіМКЅКЗHOOCCH2COOH + 2CH3CH2OH ![]() 2H2O + C2H5OOCCH2COOC2H5Ј¬
2H2O + C2H5OOCCH2COOC2H5Ј¬
№Кґр°ёОЄЈєHOOCCH2COOH + 2CH3CH2OH ![]() 2H2O + C2H5OOCCH2COOC2H5Ј»
2H2O + C2H5OOCCH2COOC2H5Ј»
ўЗEКЗ»·јєП©Ј¬Жд·ЦЧУКЅКЗC6H12Ј¬
№Кґр°ёОЄЈєC6H12Ј»
ўИёщѕЭHµДЅб№№µГіцHЦРє¬Сх№ЩДЬНЕµДГыіЖКЗхҐ»щЈ¬
№Кґр°ёОЄЈєхҐ»щЈ»
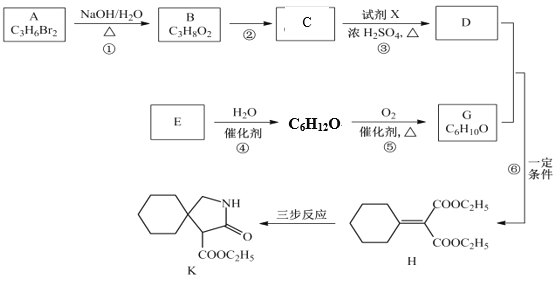
ўЙРґіцВъЧгПВБРМхјюµД KµДТ»ЦЦН¬·ЦТм№№МеµДЅб№№јтКЅЈє![]()
№Кґр°ёОЄЈє![]() Ј»
Ј»
ўКHѕИэІЅ·ґУ¦єПіЙKЈ¬ёщѕЭРЕПўТэИлЎЄCN»щНЕЈ¬
![]() + HCN
+ HCN ![]()
![]() Ј¬
Ј¬

№Кґр°ёОЄЈє
 ЎЈ
ЎЈ


 µЪ1ѕнµҐФЄФВїјЖЪЦРЖЪД©ПµБРґр°ё
µЪ1ѕнµҐФЄФВїјЖЪЦРЖЪД©ПµБРґр°ё
| Дкј¶ | ёЯЦРїОіМ | Дкј¶ | іхЦРїОіМ |
| ёЯТ» | ёЯТ»Гв·СїОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СїОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СїОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СїОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СїОіМНЖјцЈЎ | іхИэ | іхИэГв·СїОіМНЖјцЈЎ |
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїПВБРЛµ·ЁХэИ·µДКЗ( )
A.·ъАы°єЈ12(CF2Cl2)КЗјЧНйµДВИЎў·ъВ±ґъОпЈ¬ЛьУР2ЦЦН¬·ЦТм№№Ме
B.¶ФјЧ»щ±ЅјЧИ©(![]() )К№ёЯГМЛбјШЛбРФИЬТєНКЙ«Ј¬ЛµГчЛьє¬УРИ©»щ
)К№ёЯГМЛбјШЛбРФИЬТєНКЙ«Ј¬ЛµГчЛьє¬УРИ©»щ
C.деЛ®їЙТФЗш·Ц±ЅєНТТЛб
D.МЗАаКЗКіОпЧйіЙЦРµДЦШТЄІї·ЦЈ¬ТІКЗІъЙъДЬБїЧоёЯµДУЄСшОпЦК
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїУН»ЛщУГµДСХБПУРРн¶аМмИ»їуКЇіЙ·ЦЈ¬їуКЇЦРНщНщє¬УРBЎўCЎўOЎўNaЎўPЎўClµИФЄЛШЈ¬ЛьГЗФЪїЖС§СРѕїєН№¤ТµЙъІъЦРѕЯУРРн¶аУГНѕЎЈЗл»ШґрПВБРУР№ШОКМвЈє
(1)ПЦґъ»ЇС§ЦРЈ¬іЈАыУГ_________ЙПµДМШХчЖЧПЯАґјш¶ЁФЄЛШЎЈ
(2)CH3+Ўў-CH3ЎўCH3-¶јКЗЦШТЄµДУР»ъ·ґУ¦ЦРјдМеЎЈCH3+ЦРМјФЧУµДФУ»Ї·ЅКЅОЄ_________Ј¬CH3-µДїХјд№№РНОЄ_______ЎЈ
(3) NaЈ«єНNe»ҐОЄµИµзЧУМеЈ¬µзАлДЬI2(Na)______I1(Ne)(МоЎ°>Ў±»тЎ°<Ў±)ЎЈ
(4)ЗвВ±Лб(HX)µДµзАл№эіМИзНјЎЈ¦¤H1єН¦¤H2µДµЭ±д№жВЙ¶јКЗHF>HCl>HBr>HIЈ¬ЖдЦР¦¤H1(HF)МШ±рґуµДФТтОЄ__________Ј¬ґУФЧУЅб№№·ЦОцУ°П즤H2µЭ±дµДТтЛШОЄ__________ЎЈ

(5)БЧ»ЇЕрКЗТ»ЦЦДНДҐНїБПЈ¬ЛьїЙУГЧчЅрКфµД±нГж±Ј»¤ІгЎЈ
ўЩ БЧ»ЇЕрѕ§Меѕ§°ыИзНјјЧЛщКѕ:ЖдЦРКµРДЗтОЄБЧФЧУЎЈТСЦЄѕ§°ыЦРЧоЅьµДBЎўPФЧУµДѕаАлОЄapmЈ¬°ў·ьјУµВВЮіЈКэОЄNAЎЈФтБЧ»ЇЕрѕ§МеµДГЬ¶ИОЄ___________g/cm3ЎЈ(БРіцјЖЛгКЅјґїЙЈ¬І»±Ш»Їјт)
ўЪ БЧ»ЇЕрѕ§°ыСШЧЕМе¶ФЅЗПЯ·ЅПтµДН¶У°(НјТТЦР![]() ±нКѕPФЧУµДН¶У°)Ј¬УГ
±нКѕPФЧУµДН¶У°)Ј¬УГ![]() »іцBФЧУµДН¶У°О»ЦГ____ЎЈ
»іцBФЧУµДН¶У°О»ЦГ____ЎЈ

Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїIЎўИзНјЛщКѕЈ¬ёЯОВПВЈ¬і¬Сх»ЇјШѕ§МеіКБў·ЅМеЅб№№Ј¬ѕ§МеЦРСхµД»ЇєПјЫІї·ЦОЄ0јЫЈ¬Ії·ЦОЄ-2јЫЎЈИзНјОЄі¬Сх»ЇјШѕ§МеµДТ»ёцѕ§°ыЈЁѕ§МеЦРЧоРЎµДЦШёґµҐФЄЈ©Ј¬ФтПВБРЛµ·ЁЦРХэИ·µДКЗ______________ЎЈ
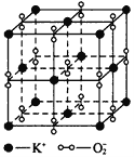
AЈ®і¬Сх»ЇјШµД»ЇС§КЅОЄKO2Ј¬Гїёцѕ§°ыє¬УР4ёцK+єН4ёцO![]()
BЈ®ѕ§МеЦРГїёцK+ЦЬО§УР8ёцO![]() Ј¬ГїёцO
Ј¬ГїёцO![]() ЦЬО§УР8ёцK+
ЦЬО§УР8ёцK+
CЈ®ѕ§МеЦРУлГїёцK+ѕаАлЧоЅьµДK+УР8ёц
DЈ®ѕ§МеЦРЈ¬0јЫСхУл-2јЫСхµДКэДї±ИОЄ3ЎГ1
IIЎўУРAЎўBЎўCИэЦЦѕ§МеЈ¬·Ц±рУЙCЎўHЎўNaЎўClЛДЦЦФЄЛШЦРµДТ»ЦЦ»тјёЦЦРОіЙЈ¬¶ФХв3ЦЦѕ§МеЅшРРКµСйЈ¬Ѕб№ыИз±нЛщКѕЈє
ПоДї | ИЫµг/Ўж | УІ¶И | Л®ИЬРФ | µјµзРФ | Л®ИЬТєУлAg+·ґУ¦ |
A | 811 | ЅПґу | ТЧИЬ | Л®ИЬТєЈЁ»тИЫИЪЈ©µјµз | °ЧЙ«іБµн |
B | 3500 | єЬґу | І»ИЬ | І»µјµз | І»·ґУ¦ |
C | -114.2 | єЬРЎ | ТЧИЬ | ТєМ¬І»µјµз | °ЧЙ«іБµн |
ЈЁ1Ј©ѕ§МеAµД»ЇС§КЅОЄ____________________ЎЈ
ЈЁ2Ј©ѕ§МеBµДѕ§МеАаРНОЄ____________________ЎЈ
ЈЁ3Ј©ѕ§МеCЦРБЈЧУјдµДЧчУГБ¦ОЄ____________________ЎЈ
IIIЎўЙй»ЇпШКЗУЕБјµД°лµјМеІДБПЈ¬їЙУГУЪЦЖЧчОўРНј¤№вЖч»тМ«СфДЬµзіШµИЎЈ
ЈЁ1Ј©Йй»ЇпШµДѕ§°ыЅб№№ИзПВНјЛщКѕЈ¬ФтЙй»ЇпШµД»ЇС§КЅОЄ____ЎЈ

ЈЁ2Ј©»щМ¬AsФЧУµДєЛНвµзЧУЕЕІјКЅОЄ_________ЎЈ
ЈЁ3Ј©µЪТ»µзАлДЬЈєGa____AsЈЁМоЎ°>Ў±»тЎ°<Ў±Ј©ЎЈ
ЈЁ4Ј©GaF3µДИЫµгёЯУЪ1 000 ЎжЈ¬GaCl3µДИЫµгОЄ77.9 ЎжЈ¬ЖдФТтКЗ_________ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїNH4Al(SO4)2КЗКіЖ·јУ№¤ЦРЧоОЄїмЅЭµДКіЖ·МнјУјБЈ¬УГУЪ±єїѕКіЖ·Ј»NH4HSO4ФЪ·ЦОцКФјБЎўТЅТ©ЎўµзЧУ№¤ТµЦРУГНѕ№г·єЎЈЗл»ШґрПВБРОКМвЈє
(1)NH4Al(SO4)2їЙЧчѕ»Л®јБЈ¬ЖдФАнКЗ__________________________________________(УГ±ШТЄµД»ЇС§УГУпєНПа№ШОДЧЦЛµГч)ЎЈ
(2)ПаН¬МхјюПВЈ¬0.1 molЎ¤LЈ1µДNH4Al(SO4)2ИЬТєєН0.1 molЎ¤LЈ1NH4HSO4ИЬТєЦРµДc(![]() )Ј¬З°ХЯ_____єуХЯЎЈ(МоЎ°µИУЪЎ±ЎўЎ°ґуУЪЎ±»тЎ°РЎУЪЎ±)
)Ј¬З°ХЯ_____єуХЯЎЈ(МоЎ°µИУЪЎ±ЎўЎ°ґуУЪЎ±»тЎ°РЎУЪЎ±)
(3)ѕщОЄ0.1 molЎ¤LЈ1µДјёЦЦµзЅвЦКИЬТєµДpHЛжОВ¶И±д»ЇµДЗъПЯИзНјЛщКѕЎЈ
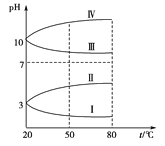
ўЩЖдЦР·ыєП0.1 molЎ¤LЈ1NH4Al(SO4)2µДpHЛжОВ¶И±д»ЇµДЗъПЯКЗ_______ (МоЧЦДё)Ј¬µјЦВpHЛжОВ¶И±д»ЇµДФТтКЗ__________________Ј»
ўЪ20 ЎжК±Ј¬0.1 molЎ¤LЈ1µДNH4Al(SO4)2ЦР2c(SO42Ј)Јc(NH4+)Ј3c(Al3Ј«)ЈЅ____________ЎЈ
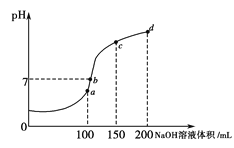
(4)КТОВК±Ј¬Пт100 mL 0.1 molЎ¤LЈ1NH4HSO4ИЬТєЦРµОјУ0.1 molЎ¤LЈ1NaOHИЬТєЈ¬ИЬТєpHУлNaOHИЬТєМе»эµД№ШПµЗъПЯИзНјЛщКѕЎЈКФ·ЦОцНјЦРaЎўbЎўcЎўdЛДёцµгЈ¬Л®µДµзАліМ¶ИЧоґуµДКЗ___________µгЈ»ФЪbµгЈ¬ИЬТєЦРёчАлЧУЕЁ¶ИУЙґуµЅРЎµДЕЕБРЛіРтКЗ_________________________ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїПВ±нОЄФЄЛШЦЬЖЪ±нµДТ»Ії·ЦЎЈ
Че ЦЬЖЪЎЎЎЎ | ўсA | ўтA | ўуA | ўфA | ўхA | ўцA | ўчA |
1 | ўЩ | ||||||
2 | ўЪ | ўЭ | |||||
3 | ўЫ | ўЮ | |||||
4 | ўЬ | ўЯ | |||||
(1)±нЦР________(МоФЄЛШ·ыєЕ)µД·ЗЅрКфРФЧоЗїЈ»________(МоФЄЛШ·ыєЕ)µДЅрКфРФЧоЗїЈ¬РґіцёГФЄЛШµДµҐЦКУлЛ®·ґУ¦µДАлЧУ·ЅіМКЅЈє___________________________________________________ЎЈ
(2)±нЦРФЄЛШўЫµДФЧУЅб№№КѕТвНјКЗ____________ЎЈ
(3)±нЦРФЄЛШўЮЎўўЯµДЗв»ЇОпµДОИ¶ЁРФЛіРтОЄ
(4)±нЦРФЄЛШЧоёЯјЫСх»ЇОп¶ФУ¦Л®»ЇОпЛбРФЧоЗїµДКЗ________(Мо»ЇС§КЅ)ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїПВБРТАѕЭИИ»ЇС§·ЅіМКЅµГіцµДЅбВЫХэИ·µДКЗ
2C(s) +O2(g) =2CO (g) ЎчH2ФтЎчH1ЈјЎчH2
A.ТСЦЄ2SO2(g)+O2![]() 2SO3(g) ОЄ·ЕИИ·ґУ¦Ј¬ФтSO2µДДЬБїТ»¶ЁёЯУЪSO3µДДЬБї
2SO3(g) ОЄ·ЕИИ·ґУ¦Ј¬ФтSO2µДДЬБїТ»¶ЁёЯУЪSO3µДДЬБї
B.ТСЦЄC(КЇД«,s)==CЈЁЅрёХКЇ,sЈ©ЎчHЈѕ0Ј¬ФтЅрёХКЇ±ИКЇД«ОИ¶Ё
C.ТСЦЄH+(aq)+OH-(aq)=H2O(1)ЎчH=Ј57.3kJЈЇmolЈ¬ФтИОєОЛбјоЦРєН·ґУ¦µДИИР§У¦ѕщОЄ57.3 kJ
D.ТСЦЄ2C(s) +2O2(g) =2 CO2(g)ЎчH1
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїПВБРУР№ШВИЛ®µДРрКцІ»ХэИ·µДКЗ(ЎЎЎЎ)
A.РВЦЖВИЛ®їЙК№pHКФЦЅПИ±дємЈ¬єуНКЙ«B.ВИЛ®·ЕЦГКэМмєуЈ¬ИЬТєµДЛбРФЦрЅҐјхИх
C.РВЦЖµДВИЛ®Ц»є¬Cl2ЎўH2OєНHClOИэЦЦ·ЦЧУD.№вХХВИЛ®УРЖшЕЭТЭіцЈ¬ёГЖшМеКЗO2
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїAЎўBЎўCИэЦ»ЙХ±ЦРЈ¬ТАґО·Ц±рКўУРNaOHИЬТєЎўKSCNИЬТєЎўЦу·РµДХфБуЛ®Ј¬ёчµОИлFeCl3ИЬТєЈ¬КФёщѕЭКµСйПЦПу·Ц±р»ШґрТФПВОКМвЈє
(1)·Ц±рРґіцИэЦ»ЙХ±ЦРРОіЙ·ЦЙўПµµДГыіЖЈє
A________Ј¬B________Ј¬C________ЎЈ
(2)РґіцCЦРРОіЙ·ЦЙўПµµД»ЇС§·ЅіМКЅЈє_____________________________________ЎЈ
(3)ИфІ»УГ»ЇС§КФјБјш±рЈ¬Рґіцјш±рBЎўC·ЦЙўПµµДБЅЦЦјтµҐ·Ѕ·ЁЈє
ўЩ______________________________________________________Ј¬
ўЪ________________________________________________________ЎЈ
(4)ПтCЦРЦрµОјУИлПЎH2SO4Ј¬ПЦПуОЄ________________________________ЎЈ
Ійїґґр°ёєНЅвОц>>
№ъјКѧУУЕСЎ - Б·П°ІбБР±н - КФМвБР±н
єю±±КЎ»ҐБЄНшОҐ·ЁєНІ»БјРЕПўѕЩ±ЁЖЅМЁ | НшЙПУРє¦РЕПўѕЩ±ЁЧЁЗш | µзРЕХ©ЖѕЩ±ЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРє¦РЕПўѕЩ±ЁЧЁЗш | ЙжЖуЗЦИЁѕЩ±ЁЧЁЗш
ОҐ·ЁєНІ»БјРЕПўѕЩ±Ёµз»°Јє027-86699610 ѕЩ±ЁУКПдЈє58377363@163.com