
����Ŀ����ѧС�������·����ⶨ��������ķ�ˮ�б��ӵĺ�������ˮ�в������Ųⶨ�����ʣ���
������ȷ������KBrO3��������һ�������a mol��L1 KBrO3����Һ��
��ȡv1 mL������Һ���������KBr����HSO4�ữ����Һ��ɫ���ػ�ɫ��
�����������Һ�м���v2 mL��ˮ��
�������������KI��
������b mol��L1 Na2S2O3����Һ�ζ�������Һ��dz��ɫʱ���μ�2�ε�����Һ�������ζ����յ㣬������Na2S2O3��Һv2 mL��
��֪��I2+2Na2S2O3=2NaI+ Na2S4O6
Na2S2O3��Na2S4O6��Һ��ɫ��Ϊ��ɫ
��1������������Һ�õ��IJ����������ձ�������������ͷ�ιܺ�____________��
��2�����з�����Ӧ�����ӷ���ʽ��_______________________________��
��3�����з�����Ӧ�Ļ�ѧ����ʽ��_________________________________��
��4�����м�KIǰ����Һ��ɫ��Ϊ��ɫ��ԭ����______________________________��
��5��KI��KBrO3���ʵ�����ϵΪn��KI����6n��KBrO3��ʱ��KIһ��������������________��
��6�����еζ����յ��������_____________________________��
��7����ˮ�б��ӵĺ���Ϊ___________g��L1������Ħ��������94 g��mol 1����
��8������Br2����____________���ʣ���~���з�Ӧ�����ܱ������н��У��������ɲⶨ���ƫ�ߡ�
���𰸡�����ƿ����Ͳ BrO3- + 5Br- + 6H+ = 3Br2 + 3H2O  �������ɵ�Br2���ˮ�б�����ȫ��Ӧ������Һ��ɫΪ��ɫ��˵����Br2ʣ�࣬ʣ��Br2�����KI��Ӧ����I2�����ú����ζ����������Ӷ���Ӽ��㱽�����ĵ�Br2 ���з�ӦΪKBrO3 + 5KBr + 3H2SO4=3K2SO4+ 3Br2 + 3H2O��֪3n(KBrO3)=n(Br2)������Br2�����뱽�ӷ�Ӧ��ʣ�����ڢ��з�ӦΪBr2+2KI=I2+2KBr����ʣ������ȫ��Ӧ����n(KI)�� 2n(Br2)����֪n(KI)��6n(KBrO3) ���������һ��Na2S2O3����Һʱ����Һ����ɫ��Ϊ��ɫ����30s����ɫ
�������ɵ�Br2���ˮ�б�����ȫ��Ӧ������Һ��ɫΪ��ɫ��˵����Br2ʣ�࣬ʣ��Br2�����KI��Ӧ����I2�����ú����ζ����������Ӷ���Ӽ��㱽�����ĵ�Br2 ���з�ӦΪKBrO3 + 5KBr + 3H2SO4=3K2SO4+ 3Br2 + 3H2O��֪3n(KBrO3)=n(Br2)������Br2�����뱽�ӷ�Ӧ��ʣ�����ڢ��з�ӦΪBr2+2KI=I2+2KBr����ʣ������ȫ��Ӧ����n(KI)�� 2n(Br2)����֪n(KI)��6n(KBrO3) ���������һ��Na2S2O3����Һʱ����Һ����ɫ��Ϊ��ɫ����30s����ɫ ![]() �ӷ�
�ӷ�
��������
���⿼��������ԭ��Ӧ�ζ����ۺ����á��������巴Ӧ�������������ζ��յ������жϣ�����Ƶ�һ��������ֱ��뱽�Ӻ�KI��Ӧ��������ȫ��Ӧ�꣩��������KI��Ӧ���ɵ�I2��Na2S2O3���еζ����������ֱ�Ӳ����KI��Ӧ�����ĵ��壬����������뱽�ӷ�Ӧ���ĵ��壬�����ݱ������巴Ӧ��ϵ�������ˮ�б��ӵ�Ũ�ȡ�
��1��ȷ����KBrO3����������Һ����IJ����������ձ�����Ͳ������������ͷ�ιܣ�һ����������ƿ������ÿ�������ƿ����Ͳ��
��2��KBrO3��Һ�м���KBr��H2SO4����Һ��ɫ���ػ�ɫ��˵������Br2������ȱ����ƽ��֪�����ӷ���ʽΪBrO3- + 5Br- + 6H+ = 3Br2 + 3H2O��
��3�����Ӻ���ˮ��Ӧ�õ���ɫ����2,4,6-���屽�ӣ���ѧ����ʽΪ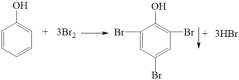 ��
��
��4���ò�������������һ��������ֱ��뱽�Ӻ�KI��Ӧ��ע�����뷴Ӧ��ȫ����һ�������������֪����������KI��Ӧ���ɵ�I2������������ԭ�ζ��������������������KI��Ӧ���������������һ�������ȥ��KI��Ӧ��������������ɵ��뱽�ӷ�Ӧ����������������һ���������뱽�ӷ�Ӧ�꣬������ʣ�������KI��Ӧ�����з�Ӧ����ʱ������Һ�Ի�ɫ˵�����ӷ�Ӧ�꣬������ʣ�࣬�Ա���KI��Ӧ����ԭ��Ϊ�������ɵ�Br2���ˮ�б�����ȫ��Ӧ������Һ��ɫΪ��ɫ��˵����Br2ʣ�࣬ʣ��Br2�����KI��Ӧ���Ӷ���Ӽ��㱽�����ĵ�Br2��
��5�����з�ӦΪKBrO3 + 5KBr + 3H2SO4=3K2SO4+ 3Br2 + 3H2O��֪3n(KBrO3)=n1(Br2)������Br2�����뱽�ӷ�Ӧ��ʣ���������Ϊn2(Br2)��n1(Br2)>n2(Br2)���ڢ��з�ӦΪBr2+2KI=I2+2KBr����ʣ������ȫ��Ӧ����n(KI)�� 2n2(Br2)����֪n(KI)��6n(KBrO3)�������n(KI)��6n(KBrO3)��KIһ��������
��6�����к������Һ�ڼ�����ۣ���Һ����ɫ������Na2S2O3��Һ���룬��ɫ��dzֱ����ʧ��������������һ��Na2S2O3����Һʱ����Һ����ɫ��Ϊ��ɫ����30s����ɫ��
��7��n(BrO3-)=av1��10-3mol�����ݷ�ӦBrO3- + 5Br- + 6H+ = 3Br2 + 3H2O��֪n(Br2)=3av1��10-3mol����ֱ��뱽�Ӻ�KI��Ӧ���ȼ�����KI���ĵ����������Ϊn1(Br2)������I2+2Na2S2O3=2NaI+Na2S4O6��֪I2~2Na2S2O3����Br2+2I-=I2+2Br-��֪Br2~ I2���ɵ�Br2~2Na2S2O3��n(Na2S2O3)= bv3��10-3mol��n1(Br2)=![]() bv3��10-3mol���ټ����ɱ������ĵ����������Ϊn2(Br2)= n(Br2)- n1(Br2)=( 3av1-
bv3��10-3mol���ټ����ɱ������ĵ����������Ϊn2(Br2)= n(Br2)- n1(Br2)=( 3av1-![]() bv3) ��10-3mol����������ˮ��Ӧ�ļ�������ϵΪ3Br2~C6H5OH��n(C6H5OH)=
bv3) ��10-3mol����������ˮ��Ӧ�ļ�������ϵΪ3Br2~C6H5OH��n(C6H5OH)=![]() n2(Br2)=(av1-
n2(Br2)=(av1-![]() bv3)��10-3mol����ˮ�б��ӵĺ���=
bv3)��10-3mol����ˮ�б��ӵĺ���=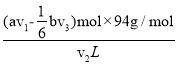 =
=![]() mol��
mol��
��8���������ɵ����뱻���Ӻ�KI��ȫ��Ӧ���������лӷ��ԣ���Ӧʱ�����ܱ������н��С�


 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�о���ѧϰС��ͬѧ����NaHCO3��KHCO3��ɵ�ij���Ȼ�������ʵ��,�����������(��������ʵ���Ũ�����):
50mL���� | 50mL���� | 50mL���� | |
m(�����) | 9.2g | 15.7g | 27.6g |
��״����,V(CO2) | 2.24L | 3.36L | 3.36L |
(1)��������ʵ���Ũ��Ϊ_________��
(2)�������,n(NaHCO3):n(KHCO3)=_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ӷ���ʽ������������Ӧʵ��������ǣ� ��
ʵ������ | ���ӷ���ʽ | |
A | ��������þ����Һ�еμ��Ȼ����Һ�������ܽ� |
|
B | ���ˮ�еμӱ����Ȼ�����Һ�õ����ɫҺ�� |
|
C | ��������ʹ���Ը��������Һ��ɫ |
|
D | ������������ϡ���� |
|
A. AB. BC. CD. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ֶ�����Ԫ��A��B��C��D����֪����C��D��ͬһ���ڣ�A��B��ͬһ���壻�����ǿ�����ɻ�����A2C��B2C2��DC2��D2A4�ȣ���B����������C�������ӵĺ�������Ų���ͬ����B2C2ͬA2C��DC2��Ӧ����������C2��B��A2C��Ӧ��������A2��A2������C2�������2��1��Ϻ��ȼ������ը���������һ�ֳ����³�������ɫ��ζ��Һ�塣��ش�
(1)д��Ԫ�ط��ţ�A________��B________��C________��D________��
(2)��A2C��B2C2��DC2��D2A4�У�ͬʱ�������Ӽ��ͷǼ��Թ��ۼ��Ļ�����ĵ���ʽΪ__________����ԭ�ӹ���ص���ʽ����Ǽ��Լ���������________��������DC2�Ľṹʽ__________��
(3)A2C���ӵĵ���ʽ____________����ԭ�ӹ���ص���ʽ���乲�ۼ���������____________��D2A4��ƽ���η��ӣ�������к���______���Ҽ���______���м���
(4)д����ѧ����ʽ�����ӷ���ʽ��
B2C2��A2C��Ӧ�Ļ�ѧ����ʽ��___________________________________________��
B2C2��DC2��Ӧ�Ļ�ѧ����ʽ��___________________________________________��
B��A2C��Ӧ�����ӷ���ʽ��______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ҩ�����ҵ�ǰ��K�ĺϳ�·�����¡�

��֪��
���� 
�����л���ṹ���ü���ʽ��ʾ����(CH3)2NCH2CH3�ļ���ʽΪ![]()
��1���л���A����Na2CO3��Һ��Ӧ����CO2�������ο�����ʳƷ�������л���B����Na2CO3��Һ��Ӧ����������CO2��B����ɵû�������A��B��Ӧ����C�Ļ�ѧ����ʽ��___________����Ӧ������___________________________��
��2��D�к��еĹ����ţ�__________________��
��3��E�Ľṹ��ʽΪ__________________��
��4��F��һ����Ȼ���ϣ�������ˮ�⡢�ữ����G��J��J����ԭ��ת��ΪG��J�Ľṹ��ʽΪ__________________��
��5��M��J��ͬ���칹�壬��������������M�Ľṹ��ʽ��__________________��
�ٰ���2����Ԫ��
��M��ˮ�⣬��NaOH��Һ����ʱ��1 mol M�������2 mol NaOH
��6���Ʋ�E��G��Ӧ�õ�K�Ĺ����У���Ӧ��LiAlH4��H2O��������__________________��
��7����K�ϳ������ҵĹ��̣�
![]()
�����Ҿ��з�ʽ�ṹ����ṹ��ʽ��__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ӦNH4Cl+NaNO2![]() NaCl+N2��+2H2O�����Ҳ������壬�����ڶ���ʯ�Ϳ��ɡ����б�ʾ��Ӧ��������Ļ�ѧ������ȷ����
NaCl+N2��+2H2O�����Ҳ������壬�����ڶ���ʯ�Ϳ��ɡ����б�ʾ��Ӧ��������Ļ�ѧ������ȷ����
A. ������Ϊ18����ԭ�ӣ�![]()
B. N2�Ľṹʽ��N=N
C. Na+�Ľṹʾ��ͼ�� 
D. H2O�ĵ���ʽ�� ![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£����и���������ָ����Һ���ܴ����������
A. 0.1 mol��L1NaOH��Һ��Na+��K+��![]() ��
��![]()
B. 0.1 mol��L1FeCl2��Һ��K+��Mg2+��![]() ��
��![]()
C. 0.1 mol��L1K2CO3��Һ��Na+��Ba2+��Cl��OH
D. 0.1 mol��L1H2SO4��Һ��K+��![]() ��
��![]() ��
��![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2001����ΰ��Ļ�ѧ�ҡ�1954��ŵ������ѧ�������������Ļ�ѧ�ṹ��ʦ��20���͵Ŀ�ѧ�ֽܱ���(L.Pauling)���ڵĵ���100���ꡣ1994����λ����л�������Ǵ����İ칫�ң�����������һ��ڰ壬���������ģ�����һ���ṹʽ��ͼ��ʾ������Ϊʲô������ṹʽ�����ܺϳ�������ʲô���ʣ����ö�֪�����DZ����������˵�һ���գ�Ҳ��������Զ������գ�Ҳ���г�һ������ܽ�������ܽ����Σ��������ȶ�����ṹ��һ���˽⡣

(1)���ķ���ʽ��__________________________________________________________��
(2)��������ԭ���Ƿ���ܴ���ͬһ��ƽ���ϣ�________(����ܡ������ܡ�)��
(3)���Ƿ���е�ɣ�________(��ǡ���)��
(4)�÷�����sp�ӻ��ĵ�ԭ����___����sp2�ӻ��ĵ�ԭ����___����sp3�ӻ��ĵ�ԭ����_____����
(5)Ϊʲô�����Ʋ�����ըҩ��______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Li4Ti5O12��LiFePO4��������ӵ�صĵ缫���ϣ���������������Ҫ�ɷ�ΪFeTiO3������������MgO��SiO2�����ʣ����Ʊ��������������£�
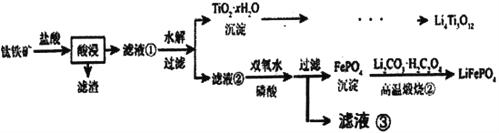
��֪���������������Ҫ��TiOCl42-��ʽ����
FeTiO3+4H++4C1-=Fe2++ TiOCl42-+2H2O
����˵������ȷ����
A. Li2Ti5O15��Ti�Ļ��ϼ�Ϊ+4��������4��������
B. ��Һ���е������ӳ���Fe2+��H+������Mg2+
C. ��Һ����Ҳ����ֱ�Ӽ���������ˮ����˫��ˮ
D. ���������բڡ������У�FeԪ�ر�����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com