
����Ŀ��������̼����������Ӧ������仯����Ľ������֮һ���ش��������⣺
(l)�ҹ������Ŷ����õ��µ�������Эͬ���������ڳ��³�ѹ��ʵ���˽�CO2��CH4һ��ת��Ϊ������Ʒ����д�� CO2��CH4�ϳ�������Ȼ�ѧ����ʽ��____��
������������ȼ���ȷֱ�Ϊ-890.31 kJ/mol��-876.72 kJ/mol��
(2)��ijһ�����ܱ�������CH4��CO2�ķ�ѹ�ֱ�Ϊ15 kPa��20 kPa������Ni/��-Al2 O3������������1123 Kʹ�䷢����Ӧ��CH4(g)+CO2(g)![]() 2CO(g)+2H2(g)��
2CO(g)+2H2(g)��
�������CO����������v��CO��=1.28![]() 10-2�qp��CH4��
10-2�qp��CH4��![]() p��CO2����kPa
p��CO2����kPa![]() s-1����ijʱ�̲��p(H2)=10 kPa���� p��CH4��=___kPa��v��CO��=___kPa
s-1����ijʱ�̲��p(H2)=10 kPa���� p��CH4��=___kPa��v��CO��=___kPa![]() s-1��
s-1��
�ڴﵽƽ�������ϵѹǿ����ʼʱ��![]() ����÷�Ӧ��ƽ�ⳣ��Kp=____kPa��2��
����÷�Ӧ��ƽ�ⳣ��Kp=____kPa��2��
(3)������(GaN)��Cu�������ͼ��ʾ���˹����ϵͳ����װ������CO2��H2OΪԭ�Ϻϳ�CH4��
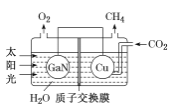
�ٸ�װ�ù���ʱH+����____���GaN����Cu�����缫���õ缫�ϵĵ缫��ӦʽΪ ___��
�ڸ�װ��ÿ����1 mol CH4��������Һ��������____g��
�۱�ʵ�������£���CO2ת��Ϊ��������顢��ϩ�ȣ���ת����Ϊ10%������CH4��ѡ����Ϊ12%�����ռ���12 mol CH4����ͨ���CO2Ϊ____mol������֪��ѡ����=����Ŀ��������ĵ�ԭ������ԭ���ܵ�ת������
(4)�����˹����ϵͳװ��Ҳ�����Ʊ���ϩ����Ȳ����Ҫ����ԭ�ϡ�2010��Sheth���о��ó���Ȳ��Pd����ѡ�����ķ�Ӧ����������ͼ��ʾ������������Pd����������á�*����ע��
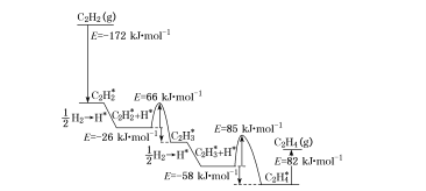
����������ӦΪ ____����ȡ����ȡ�����Ӧ���ù�������С���ݣ���ܣ�Ϊ___ kJ![]() mol-1���ò���Ļ�ѧ����ʽΪ____��
mol-1���ò���Ļ�ѧ����ʽΪ____��
���𰸡�CH4��g��+CO2��g����CH3COOH��l����H=-13.59 kJ/mol 10 1.92 3200 Cu CO2+8e-+8H+=CH4+2H2O 72 1000 ���� 66 C2H2*+H*=C2H3*
��������
��1�����ݼ���������ȼ���ȵó��Ȼ�ѧ����ʽΪ���� CH4��g��+2O2��g����CO2��g��+2H2O��l�� ��H=-890.31 kJ/mol���� CH3COOH��l��+2O2��g��=2CO2��g��+2H2O��l�� ��H=-876.72 kJ/mol �����ݸ�˹���ɢ�-�ڵ�CH4��g��+CO2��g���TCH3COOH��l����H=-890.31 kJ/mol-(-876.72 kJ/mol)=-13.59 kJ/mol����CO2��CH4�ϳ�������Ȼ�ѧ����ʽΪ��CH4��g��+CO2��g����CH3COOH��l����H=-13.59 kJ/mol���ʴ�Ϊ��CH4��g��+CO2��g����CH3COOH��l����H=-13.59 kJ/mol��
��2������ijһ�����ܱ�������CH4��CO2�ķ�ѹ�ֱ�Ϊ15 kPa��20 kPa������Ni/��-A12 O3������������1123 Kʹ�䷢����Ӧ��CH4(g)+CO2(g)![]() 2CO(g)+2H2(g)����
2CO(g)+2H2(g)����
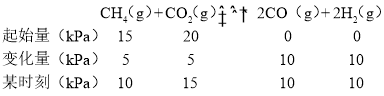
p��CH4��=10kPa��v��CO��=1.28![]() 10-2 p��CH4��
10-2 p��CH4��![]() p��CO2����kPa
p��CO2����kPa![]() s-1��=1.28
s-1��=1.28![]() 10-2��10��15=1.92��kPa
10-2��10��15=1.92��kPa![]() s-1�����ʴ�Ϊ��10��1.92��
s-1�����ʴ�Ϊ��10��1.92��
�ڴﵽƽ�������ϵѹǿ����ʼʱ��![]() ����ﵽƽ��״̬���ļ����ѹx��
����ﵽƽ��״̬���ļ����ѹx��
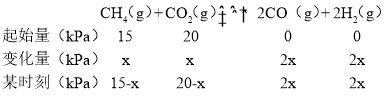
15-x+20-x+2x+2x=��15+2����![]() �����x=10�����ƽ�ⳣ��Kp=
�����x=10�����ƽ�ⳣ��Kp=![]() ���ʴ�Ϊ��3200��
���ʴ�Ϊ��3200��
��3������ͼ��֪��������ˮʧȥ�������������������϶�����̼�õ��������ɼ��飬�������������ƶ��� �����ĵ缫��ӦΪ��CO2+8e-+8H+=CH4+2H2O���ʴ�Ϊ��Cu��CO2+8e-+8H+=CH4+2H2O��
�ڸ���������Ӧʽ֪ÿ����1mol CH4ת�Ƶ�����Ϊ8mol��������Һ�������ٵ���ˮ�����������ݵ���ת���غ㼰�缫��ӦʽH2O-4e-=O2��+4H+�ã�m(H2O)=18g/mol��![]() =72g���ʴ�Ϊ��72��
=72g���ʴ�Ϊ��72��
���������n��CO2����10%��12%=12mol����n��CO2��=1000mol���ʴ�Ϊ��1000��
��4������ͼʾ֪��������Ӧ�������ͣ���ӦΪ���ȣ�����ͼʾ��Ӧ����С���ݣ���ܣ�Ϊ66 kJ![]() mol-1���ò���Ļ�ѧ����ʽΪ��C2H2*+H*=C2H3*���ʴ�Ϊ�����ȣ�66��C2H2*+H*=C2H3*��
mol-1���ò���Ļ�ѧ����ʽΪ��C2H2*+H*=C2H3*���ʴ�Ϊ�����ȣ�66��C2H2*+H*=C2H3*��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ƣ�NaClO2����һ�ָ�Ч��Ư���������������������ƣ�NaClO3��Ϊԭ����ȡ����������ClO2Ϊ��̬��������˵���������
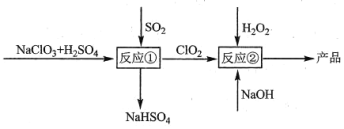
A.��Ӧ�ٽΣ��μӷ�Ӧ��NaClO3��SO2�����ʵ���֮��Ϊ2:1
B.��Ӧ�ٺ����ɵ�����Ҫ��������뷴Ӧ��װ��
C.�����¶ȣ������ڷ�Ӧ����߲���
D.��Ӧ��������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪ M��N �Ǻϳ�ij���ܸ߷��Ӳ��ϵ��м������й��� M��N ˵����ȷ����
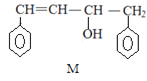
![]()
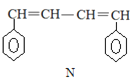
A.M��N �����ڷ����������Ȳ���ͬϵ�Ҳ����ͬ���칹��
B.M��N �ֱ���Һ���ϣ�������ȡ����Ӧ
C.M��N ����ʹ���Ը��������Һ��ɫ
D.M��N ��������ԭ�Ӿ����ܹ�ƽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������������ˮԴ�ͷ�ˮ���������У�������ȥ����Ⱦ����°���ѧ��Ⱦ����Ҳ������κ��ձ��°��IJ�����и߶ȵİ�ȫ�ԣ��������ˮ������Ũ KOH ��Һ������ǿ������Һ�б� ���ȶ���ʵ�����ô��������������Ʊ��������(K2FeO4)��������ͼ��ʾ��
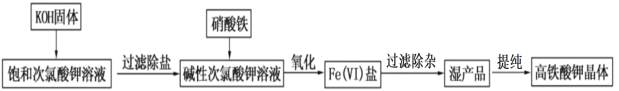
����ͼ�еı��ʹ��������Һ���Ʊ�װ����ͼ��ʾ��
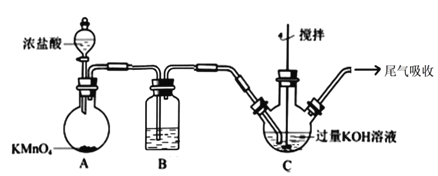
(1)A Ϊ��������װ�á�A �з�Ӧ�����ӷ���ʽ��___________��װ�� B �ڵ��Լ���������____________��
(2)װ�� C ��Ӧ�¶ȿ����� 0��5����У��ڲ��ı� KOH ��ҺŨ��ǰ���£�ʵ���пɲ�ȡ��ʩ��________��
(3)����ͼ���ڼ��� KOH �����ʱ�����������İ�ɫ����_____(�ѧʽ)��
(4)�ڽ����£��� Fe(NO3)3������Һ�����μӵ� KClO ������Һ�м�����ȡ K2FeO4��д���÷�Ӧ�����ӷ���ʽ__________����Ӧ�������¶ȿ����� 10~15�棬�¶ȹ��ͻ���߶Է�Ӧ��Ӱ����_____________��
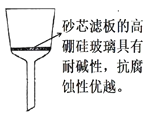
(5)���˳���ʱ������ͨ©������ֽ������ɰо©��(��ͼ)��ԭ����_____________��
(6)���ᴿ�������ʱ�����ؽᾧ��ϴ�ӡ�����ķ�����ϴ�Ӽ�����ѡ��_____��
A.H2O B.Ũ KOH ��Һ C.Fe(NO3)3��Һ D.�����
(7)���ᴿ�õ� a gK2FeO4(M=198g/mol)�������������� 5%�� Fe ��ʧ������Ҫ�ṩ Fe(NO3)3(M=242g/mol)������Ϊ_____g(�ú� a �ļ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͨ�ɵ����һ�ֹ㷺ʹ�õĿ��ƶ���ѧ��Դ�� ijͬѧ��̽�������Ըɵ�������ʻ�������ʱ��������ͼ��ʾʵ�飺
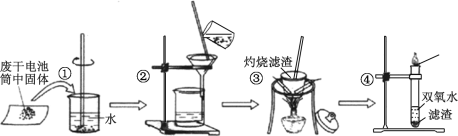
��ش��������⣺
��1���ɵ�ع���ʱ�����缫��ӦʽΪ��2NH4��+2e- = 2NH3��+H2�������缫��Ӧʽ��___________�� ����м��� MnO2 �������dz�ȥ�����ϵ�ij��� ��������Mn2O3���÷�Ӧ�Ļ�ѧ����ʽ��_______________��
��2���������������ʱ������Ҫ�����оƾ��ơ���������______ �������Ǻ����żܣ���������ʱ������һ����ɫ��ζ��ʹ����ʯ��ˮ����ǵ����壬�ɴ��Ʋ�����ǰ�������д��ڵ�������_____��
��3������ܵ��Թ��м��벽������պ����ú�ɫ���壬�Թ���Ѹ�ٲ�����ʹ�����ǵ�ľ����ȼ�����壬�ݴ˿ɳ����϶����պ�ĺ�ɫ����Ϊ_____��
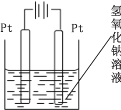
��4����ѯ���ϵ�֪��ͨ�����ɽ� Mn2O3 ת��Ϊ MnO2�� Ϊ��ijͬѧ�������ͼ��ʾװ�ã�����������ĩ���ɺ�״���������е�_________������ҡ����ߵ缫�ϣ��õ缫�Ϸ�����Ӧ�ĵ缫��Ӧʽ��_____�� �ڵ���������Һ�� pH ��__________����������С�����䡱����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����
A.C60 �� C70��Ϊͬλ��
B.N2 ��N3- ��N4��N5+��Ϊͬ��������
C.�����飨![]() ���ͱ���ϩ��Ϊͬ���칹��
���ͱ���ϩ��Ϊͬ���칹��
D.CH3CH2COOH ��HCOOCH2CH2CH3 ��Ϊͬϵ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ӷ���ʽ����ȷ����
A.�ð�ˮ���չ����Ķ�������![]()
B.��0.5mol L-1 KAl(SO4)2��Һ�е���0.5mol L-1 Ba(OH)2��Һʹ![]() ǡ����ȫ������2Ba2+ 4OH- + Al3++2
ǡ����ȫ������2Ba2+ 4OH- + Al3++2![]() =2BaSO4��+
=2BaSO4��+![]() +2H2O
+2H2O
C.������Na2S2O3��Һ��ȥˮ�е� Cl2: 4Cl2+![]() +5H2O =10H++2
+5H2O =10H++2![]() +8Cl-
+8Cl-
D.����CO2ͨ�뱽������Һ�У�C6H5O-+CO2+H2O��C6H5OH+![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ϊֹ�ҹ���ز����Ѿ�������113��Nh��115��Mc��116��Lv��117��Ts��118��Og��Ԫ�ص��������Ʒֱ��ǣ�![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() �������й��ƶ���ȷ���ǣ� ��
�������й��ƶ���ȷ���ǣ� ��
A.������Ԫ�ؾ�Ϊ����Ԫ��
B.Nh�������������һ�ֵ��͵�����������
C.Ts�ļ������ӱȸ�����һ���ڼ������ӵĻ�ԭ����
D.���ݸ����ƣ����ڱ�118��Ԫ���зǽ���Ԫ�ع���24��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��п����Ԫ���γɵĻ������ڸ�����������Ҫ�����á�
(1)��̬Zn2+�ļ۵����Ų�ʽΪ_______________��
(2)������NH3�����У�H-N-H������Ϊ107��18������ͼ��[Zn(NH3)6]2���IJ��ֽṹ�Լ�����H-N-H�����ǡ�
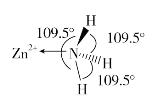
�����[Zn(NH3)6]2��������H-N-H���DZ�Ϊ109.5����ԭ����_____________��
(3)����Һ����кܸߵ�Ӧ�ü�ֵ������EMIM��������H��C��N����Ԫ����ɣ���ṹ��ͼ��ʾ��
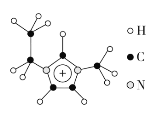
���������÷�����![]() ��ʾ������m��n�ֱ���������γɴ�������ԭ�����͵���������EMIM�������еĴ�����Ӧ��ʾΪ___________________��������[EMIM][AlCl4]���кܸߵ�Ӧ�ü�ֵ�����۵�ֻ��7 �棬�����ʾ����������________��
��ʾ������m��n�ֱ���������γɴ�������ԭ�����͵���������EMIM�������еĴ�����Ӧ��ʾΪ___________________��������[EMIM][AlCl4]���кܸߵ�Ӧ�ü�ֵ�����۵�ֻ��7 �棬�����ʾ����������________��
(4)����Ԫ��ˮ�������Ƿ�����ɫ��ԭ�ӽṹ�йأ��Ҵ���һ���Ĺ��ɣ���֪Zn2���ȹ���Ԫ�������γɵ�ˮ�����ӵ���ɫ���±���ʾ��
���� | Sc3�� | Cr3�� | Fe2�� | Zn2+ |
ˮ�����ӵ���ɫ | ��ɫ | ��ɫ | dz��ɫ | ��ɫ |
�����ԭ�ӽṹ�Ʋ�Sc3����Zn2����ˮ������Ϊ��ɫ��ԭ��____________________��
(5)Zn��S�γ�ij�ֻ�����ľ�����ͼ��ʾ��
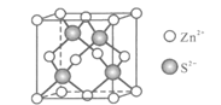
��Zn2+����S2����ɵ�________________��϶�У�
���ɢ��ܷ��жϳ�S2����Zn2+���У�_________������������������������֪�����ܶ�Ϊdg/cm3��S2���뾶Ϊa pm����ҪʹS2-��Zn2+���У���Zn2+�뾶Ϊ____________________pm��д�������ʽ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com