
����Ŀ��Fe2O3��Cu2O���Ǻ�ɫ��ĩ,����������.ʵ��С��ͨ��ʵ����̽��ijһ��ɫ��ĩ��Fe2O3��Cu2O�ĺ���(������������)��
��֪��Cu2O��H2SO4��Cu��Cu SO4��H2O
̽���������£�
ȡ�����÷�ĩ���� ����ϡ�����У���ĩ��ȫ�ܽ⡣
��1�������μ�KSCN�Լ�����Һ����Ϊ��ɫ����Fe2O3��Cu2O�����ʵ���֮��Ϊ________��
��ʵ��С�鷢�ֿ�ͨ���Է�Ӧ����Һ�ij���������̷����壨FeSO4��7H2O��������˼�����������̷����Ʊ���
���Һ![]() ����
����![]() һϵ�в���
һϵ�в���![]() �̷����壨FeSO4��7H2O��
�̷����壨FeSO4��7H2O��
�Լ�aΪ_____________���ѧʽ������һϵ�в�����Ϊ__________��__________�����ˡ�ϴ�ӡ����
��2���ⶨCu2O��������������ȡһ����Fe2O3��Cu2O��ɵĹ�������9.28g��������ڿ����г�ּ��ȣ�ֻ����Cu2O��CuO�ķ�Ӧ�������������ٱ仯ʱ���������Ϊ9.60g�������������Cu2O����������Ϊ_________________��������λ��Ч���֣�
���𰸡�1��1 Fe ����Ũ�� ��ȴ�ᾧ 31.0%
��������
��1���ٵμ�KSCN�Լ�����Һ����Ϊ��ɫ������Fe2O3+6H+=2Fe3++3H2O��Cu2O+2H+=Cu+Cu2++H2O��2Fe3++Cu=2Fe2++Cu2+��
�ڻ���̷����壨FeSO47H2O�����Լ�aΪFe���ɳ�ȥ�����ӣ�����Ũ������ȴ�ᾧ�����˷�������壻
��2����ּ���ֻ����Cu2O��CuO�ķ�Ӧ����������ΪOԪ�ص��������Դ˼��㡣
��1���ٵμ�KSCN�Լ�����Һ����Ϊ��ɫ������Fe2O3+6H+=2Fe3++3H2O��Cu2O+2H+=Cu+Cu2++H2O��2Fe3++Cu=2Fe2++Cu2+����2Fe3++Cu=2Fe2++Cu2+ǡ�÷�Ӧ����Fe2O3��Cu2O�����ʵ���֮��Ϊ1��1��
�ʴ�Ϊ��1��1��
�ڻ���̷����壨FeSO47H2O�����Լ�aΪFe���ɳ�ȥ�����ӣ������̿�֪ϵ�в���Ϊ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ����
�ʴ�Ϊ��Fe������Ũ������ȴ�ᾧ��
��2����ּ���ֻ����Cu2O��CuO�ķ�Ӧ����ԭ���غ��֪Cu2O��2CuO��O����������9.60g-9.28g=0.32g����֪n��Cu2O��=0.32g/16g/mol=0.02mol������������Cu2O����������Ϊ(0.02mol��144g/mol)/9.28g��100%=31.0%��
�ʴ�Ϊ��31.0%��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ͻ�ѧʵ������Ч������Ⱦ����ԼҩƷ����ͼ�У�ijѧ���ڳ���һ�Ű�ֽ�IJ���Ƭ�Ϸ��ñ������ڱ������ϵIJ�ͬλ�÷ֱ�μ�Ũ��Ϊ0.1mol/L��KBr��KI����������Һ����NaOH(����̪)��FeCl2(��KSCN)��Һ��1�Σ��ڱ��������Ĵ�����2С��KMnO4���壬���μ�һ��Ũ���ᣬ������������Ǻá��ɼ�KMnO4����ܿ��ܽ⣬����������
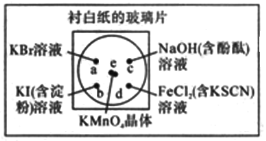
(1)��д����ѧʵ��������MnO2��ȡCl2�Ļ�ѧ����ʽ______________________________��
����ɱ�ʵ������ȡCl2�Ļ�ѧ����ʽ��
___________KMnO4+__________HCl(Ũ)����______KCl+________MnCl2+____Cl2��+______ _______
��÷�Ӧ�����Ļ�ԭ�������ʵ���Ϊ8mol�������ת����ĿΪ_________________��
(2)b����ʵ������Ϊ____________________________________________________��
c����ʵ������Ϊ____________________________________________________��
(3)d����Ӧ�����ӷ���ʽΪ____________________��____________________��
(4)ͨ����ʵ���ܱȽ�Cl2��FeCl3��KMnO4�������������Ե�ǿ��������������ǿ������˳����_________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��AgNO3��ˮ��Һ��________________�����������������������������ԣ�����ʱ��pH_____7������>������=������<������ԭ���ǣ������ӷ���ʽ��ʾ����_______________________________________________________________________________________��
ʵ����������AgNO3����Һʱ������AgNO3���������ڽ�Ũ�������У�Ȼ����������ˮϡ�͵������Ũ�ȣ���____________�������ٽ�����������������ˮ�⡣
��2���Ȼ���ˮ��Һ��_______�� ��ԭ���ǣ������ӷ���ʽ��ʾ����_____________________________________________________________ ��
��AlCl3��Һ���ɣ����գ����õ�����Ҫ���������____________________��
��3��������������Һʱ��Ϊ�˷�ֹ����ˮ�⣬���Լ���������______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�������A��������������������ܶ���42��������ױ��������к���̼̼˫�����˴Ź������ױ���������ֻ��һ�����͵��⡣
��1��A�Ľṹ��ʽΪ____������Ϊ____��
��2��A���ж���̼ԭ�Ӵ���ͬһƽ��____��
��3����֪A��B��C����ͼת����ϵ��
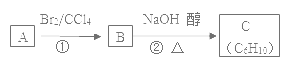
��Ӧ�ڵĻ�ѧ����ʽΪ___����Ӧ��������____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��1��2 �� ��������������Ϳ����������Ӽ���������������ɫҺ��,�ܶ�2.18 g��cm-3���е�131.4�棬�۵�9.79�棬������ˮ�������ڴ����ѡ���ͪ���л��ܼ�����ʵ�����п�������ͼ��ʾװ���Ʊ�1��2���������飬���з�Һ©������ƿa��װ���Ҵ���Ũ����Ļ��Һ���Թ�d��װ��Һ��(���渲������ˮ)��

��д���пհף�
(1)д���������Ʊ�1��2�����������������ѧ��Ӧ����ʽ��
___________________________________________________________
___________________________________________________________��
(2)��ȫƿb���Է�ֹ�����������Լ��ʵ�����ʱ�Թ�d�Ƿ�����������д����������ʱƿb�е�����_________________________________��
(3)����c��NaOH��Һ�������ǣ�__________________________________��
(4)ijѧ��������ʵ��ʱ��ʹ��һ������Һ�壬����ȫ����ɫʱ���������Ҵ���Ũ������Һ��������������³������࣬���װ�õ�������û�����⣬�Է�������ܵ�ԭ��_______________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�Ƽ��ƵõĴ����к���NaHCO3��NaCl���ʡ�ijʵ��С��Դ˼���Ʒ���м�顣
��1��ȡ�˼���Ʒ10g�����ȵ��������ټ��٣���ȴ���أ��Ƶ�����Ϊ9.845g������Ʒ��NaHCO3����������Ϊ__________________________��
��2����ȡ10g������Ʒ��������ˮ�ܽ⣬���250mLһ�����ʵ���Ũ�ȵ���Һ�����ƹ����б���ʹ�õĻ�ѧ������______________________������ĸ��
A.�ձ� B.250mL����ƿC.©�� D.��ͷ�ι� E.������ F.�Թ�
��3����ȡ�������ƺõ���Һ25mL�������м������������ ���ٲ������ݣ����ռ���190.4mL����״�������壬�÷�Ӧ���̵����ӷ���ʽΪ______________________��_______________________��10g����Ʒ�к�____________gNaCl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ�ϳ����ú����ˮ����Na2S2O35H2O��ʵ���ҿ�������װ�ã���ȥ���ּӳ�������ģ�����ɹ��̡�
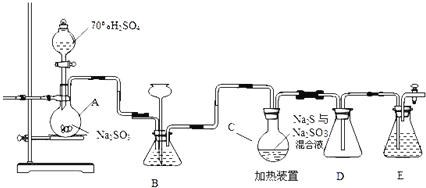
��ƿC�з�����Ӧ���£�
Na2S��aq��+H2O��l��+SO2��g��=Na2SO3��aq��+H2S��aq������ ����
2H2S��aq��+SO2��g��=3S��s��+2H2O��l�������������������� ����
S��s��+Na2SO3��aq��![]() Na2S2O3��aq������������������ ����
Na2S2O3��aq������������������ ����
��1��װ��A�з����Ļ�ѧ��Ӧ����ʽΪ ______
��2��Ϊ��߲�Ʒ���ȣ�Ӧʹ��ƿC��Na2S��Na2SO3ǡ����ȫ��Ӧ������ƿC��Na2S��Na2SO3���ʵ���֮��Ϊ ______ ��
��3��װ��B������֮һ�ǹ۲�SO2���������ʣ����е�Һ�����ѡ�� ______
a������ˮ����b������Na2SO3��Һ����c������NaHSO3��Һ�� d������NaHCO3��Һ
ʵ���У�ΪʹSO2����������ƿC�����õIJ����� ______ ��
��4����֪��Ӧ������Խ���������ƿC�з�Ӧ�ﵽ�յ�������� ______ ��װ��E������Ϊ ______
��5����Ӧ��ֹ����ƿC�е���Һ������Ũ����������Na2S2O35H2O�����п��ܺ���Na2SO3��Na2SO4�����ʣ����������Լ����ʵ�飬����Ʒ���Ƿ����Na2SO4����Ҫ˵��ʵ�����������ͽ��ۣ� ______ ��
��֪��Na2S2O35H2O�����ֽ⣺S2O32+2H+=S��+SO2��+H2O����ѡ����Լ���ϡ���ᡢϡ���ᡢϡ���ᡢBaCl2��Һ��AgNO3��Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������к�ǿ�������ԣ���ҵ�ϱ�������ͨ��ʹ�ø���ĸ�ƿ�������ƿ��λ�÷���©������©��������ʱ�����ǿ���ʹ��Ũ��ˮ�����顣�����й�˵������ȷ����(�� ��)
A.��ƿʢװ����ǰ����δ�ܸ��������������2Fe + 3Cl2 = 2FeCl3 ��Ӧ����
B.��֪�����백ˮ��ӦΪ8NH3 + 3Cl2 = N2 + 6NH4Cl ��ÿ��1mol Cl2�μӷ�Ӧ��ת�Ƶ���Ϊ2mol
C.��������ʱӦ��ʹ�ý����ܵ����߿���ʴ��߷��Ӳ��Ϲܵ�
D.����ˮʹ��������������������Ϊ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ӷ���ʽ��ȷ����
A.��Fe(NO3)2ϡ��Һ�м������3Fe2����4H����NO3-===3Fe3����NO����2H2O
B.̼�������Һ��������NaOH��Һ��Ϻ���ȣ� NH4+��OH��![]() NH3����H2O
NH3����H2O
C.������KHSO4��Һ��Ba(OH)2��Һ��Ӧ��Ba2����OH����H����SO42��===BaSO4����H2O
D.��ϡ����������Һ��ͨ������CO2��OH����CO2===HCO3-
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com