
����Ŀ����֪������������ճ������м�Ϊ�������ᣬ��һ�������£�CH3COOH��Һ�д��ڵ���ƽ�⣺CH3COOH![]() CH3COO+H+ ��H��0��
CH3COO+H+ ��H��0��
��1�����³�ѹ�£���pH=5��ϡ������Һ�У�c(CH3COO��=__������ȷֵ�����з����У�����ʹ0.10mol��L��1CH3COOH�ĵ���̶��������___��
a.��������0.10mol��L1��ϡ���� b.����CH3COOH��Һ c.�������������� d.��ˮϡ����0.010mol��L��1 e.���������Ȼ��ƹ��� f.��������0.10mol��L��1��NaOH��Һ
��2����֪��90��ʱ��ˮ�����ӻ�����ΪKw=38��10��14���ڴ��¶��£���pH=3�������pH=11������������Һ�������ϣ�������Һ�е�c(H+)=___��������λ��Ч���֣���
��3������Ũ�Ⱦ�Ϊ0.1mol/L��������Һ�������� �ڴ��� ���������� ���Ȼ�� �ݴ���� ������� ��������� �ఱˮ����ش��������⣺
��.�١��ڡ��ۡ���������Һ����ˮ�������H����Ũ���ɴ�С��˳����(�����)__��
��.�ܡ��ݡ��ޡ��ߡ���������Һ��NH4+Ũ���ɴ�С��˳����(�����)___��
��4��һ�������¼״���һ����̼��Ӧ���Ժϳ����ᡣ���������£���a mol/L��CH3COOH��b mol/LBa��OH��2��Һ�������ϣ���Ӧƽ��ʱ��2c��Ba2+��=c��CH3COO-�����ú�a��b�Ĵ���ʽ��ʾ�û����Һ�д���ĵ��볣��Ϊ___��
���𰸡���10��5��10��9��mol��L��1 bdf 2.05��10��11mol��L-1 �ܢڢۢ� �ޢߢܢݢ� ![]()
��������
��1����Һ��H�����������������Ļ���ˮ��������ġ�����ˮ�����ӻ���֪��ˮ���������c��H����=c��OH����=![]() =10��9mol��L��1����˴�����������c��H����=c��CH3COO����=��10��5��10��9��mol��L��1��������ڵĵ���ƽ��ΪCH3COOH
=10��9mol��L��1����˴�����������c��H����=c��CH3COO����=��10��5��10��9��mol��L��1��������ڵĵ���ƽ��ΪCH3COOH![]() CH3COO����H������
CH3COO����H������
a������������������Һc(H��)���Դ���ĵ������������ã�a����
b��������ʵĵ��������ȷ�Ӧ�������¶ȣ�����������뷽����У�b��ȷ��
c����������ᣬ����̶Ƚ��ͣ�c����
d����ˮϡ�ͣ�������Ũ�ȣ�������������ԭ����ƽ����Ũ������ķ�����У�������뷽����У�d��ȷ��
e�������Ȼ��ƹ��壬�Ե�����Ӱ�죬e����
f�������������ƣ�����H������ʹƽ������뷽����У�f��ȷ���ʴ�ѡbdf��
��2��pH=3��������c��H+��=10-3mol/L��pH=11������������Һ��c��OH-��=![]() =38��10-3mol/L����Ϻ�H++OH-=H2O�����Լ�ʣ�࣬ʣ�����������Ũ��Ϊc��OH-��=
=38��10-3mol/L����Ϻ�H++OH-=H2O�����Լ�ʣ�࣬ʣ�����������Ũ��Ϊc��OH-��=![]() =0.0185mol/L������c��H+��=
=0.0185mol/L������c��H+��=![]() ��2.05��10-11 mol��L-1��
��2.05��10-11 mol��L-1��
��3����.����ʹ��ᡢ�������ƾ�����ˮ�ĵ��룬��Ũ�ȵ�����ʹ��ᡢ������������Һ�е������H+��OH-Ũ�ȴ�С��ϵ��H2SO4��NaOH��CH3COOH����˶�ˮ�ĵ�����������ǿ����ϵΪ��H2SO4��NaOH��CH3COOH����ˮ���������H+Ũ�ȴ�С��ϵ��CH3COOH��NaOH��H2SO4������ΪNH4Cl�ܹ�ˮ�⣬��ˮ�ĵ�����ٽ����ã�����������Һ����ˮ�������H+Ũ���ɴ�С��˳��NH4Cl��CH3COOH��NaOH��H2SO4�����ܢڢۢ١�
��.�Ȼ�狀��������Һ�д�����NH4Cl=NH4++Cl-��NH4++H2O![]() NH3
NH3![]() H2O+H+���������Һ��CH3COONH4=CH3COO-+NH4+��CH3COO-+H2O
H2O+H+���������Һ��CH3COONH4=CH3COO-+NH4+��CH3COO-+H2O![]() CH3COOH+OH-��NH4++H2O
CH3COOH+OH-��NH4++H2O![]() NH3
NH3![]() H2O+H+�����������Һ��NH4HSO4=NH4++H++SO42-��NH4++H2O
H2O+H+�����������Һ��NH4HSO4=NH4++H++SO42-��NH4++H2O![]() NH3
NH3![]() H2O+H+����ˮ��NH3
H2O+H+����ˮ��NH3![]() H2O
H2O![]() NH4++OH-����Ũ�ȵ��������ʣ��������NH4+�ij�ʼŨ������Ȼ����Һ���������Һ�����������Һ��NH4+�ij�ʼŨ����ͬ���������CH3COO-ˮ�����ɵ�OH-��NH4+��ˮ����ٽ����ã����������H+��NH4+��ˮ�����������ã�������֮��NH4+Ũ�ȴ�СΪ��NH4HSO4��NH4Cl��CH3COONH4������Ϊ��ˮֻ�ܲ��ֵ��룬����������Һ�к��е�NH4+Ũ����С����������������Һ��NH4+Ũ���ɴ�С��˳���ǣ��ޢߢܢݢࣻ
NH4++OH-����Ũ�ȵ��������ʣ��������NH4+�ij�ʼŨ������Ȼ����Һ���������Һ�����������Һ��NH4+�ij�ʼŨ����ͬ���������CH3COO-ˮ�����ɵ�OH-��NH4+��ˮ����ٽ����ã����������H+��NH4+��ˮ�����������ã�������֮��NH4+Ũ�ȴ�СΪ��NH4HSO4��NH4Cl��CH3COONH4������Ϊ��ˮֻ�ܲ��ֵ��룬����������Һ�к��е�NH4+Ũ����С����������������Һ��NH4+Ũ���ɴ�С��˳���ǣ��ޢߢܢݢࣻ
��4�����������£���a mol/L��CH3COOH��b mol/L Ba(OH)2��Һ�������ϣ�������Һ���ֵ����ԣ�2c(Ba2��)+c(H+)��c(CH3COO��)+c(OH��)����2c(Ba2��)��c(CH3COO��)��b mol/L������Һ�������ԣ���c(H+)=c(OH��)=1��10-7mol/L�����ݴ���������������Һ��Ӧ�Ļ�ѧ����ʽȷ�����Һ��c(CH3COOH)=![]() mol/L���û����Һ�д���ĵ��볣��Ϊ
mol/L���û����Һ�д���ĵ��볣��Ϊ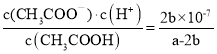 ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ԭ���ԭ���ķ��ֺ��ֵ�صķ������ı������ǵ����ʽ����ͼ��ʾԭ���װ�õ����˵���У�����ȷ����
A.�ܽ���ѧ��ת��Ϊ����
B.���Ӵ� Cu ���������� Zn
C.��Һ��H+��![]() �ֱ���ͭ��п�缫�ƶ�
�ֱ���ͭ��п�缫�ƶ�
D.��װ���з������ܷ�ӦΪ�� Zn + 2H+ = Zn2+ + H2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
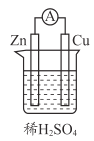
����Ŀ����ͼ��ʾ�Dz��ֶ�����Ԫ��ԭ�Ӱ뾶��ԭ�������Ĺ�ϵ ���ش���������
(l)N�����ڱ��е�λ���� _______________��
(2)X��Y��P��Q ����Ԫ�ص�����������ˮ�����У���ѧ�������Բ�ͬ���������ֵ������� ______________��
(3)�õ���ʽ��ʾ������MQ ���γɹ���_______________��
(4)Y����������Ӧ��ˮ�����ϡ��Һ����� Cu ��Ӧ�Ļ�ѧ����ʽ��________��
(5)Ԫ�� X ����Ԫ�ؿ����γɶ��ֻ������ͼģ�ͱ�ʾ�ķ����У��������� X ����Ԫ���γɵ���____________(�����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ȼ��Դ�Ʊ���ػ�ѧ���ʣ����л�ѧ��������������
A.��ȡʳ�κ�ĸҺ![]() ��Br2��Һ��
��Br2��Һ��![]()
![]() ����
����![]() ����
����
B.ʯӢɰ![]() �ֹ�
�ֹ�![]() �����Ȼ���
�����Ȼ���![]() �ߴ���
�ߴ���
C.FeS2![]() SO2
SO2![]() H2SO3
H2SO3![]() H2SO4
H2SO4
D.������![]() Na[Al(OH)4]��Һ
Na[Al(OH)4]��Һ![]() Al(OH)3
Al(OH)3![]() Al2O3
Al2O3![]() ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ƾ���(Na2S2O3��5H2O)���������մ������ֳ�������������֪��������ˮ���������Ҵ��������ֽ⣬���н�ǿ�Ļ�ԭ�ԣ��㷺��Ӧ��������ȹ�ҵ�С�ijʵ����ģ�ҵ���ȡ��������ƣ��䷴Ӧװ�ü������Լ���ͼ��
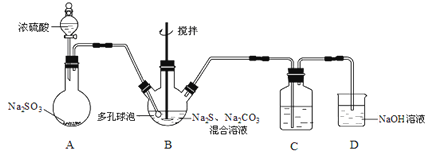
(1)װ��A�з�����Ӧ�Ļ�ѧ����ʽ��_____________��
(2)װ��B��ͨ��SO2��Ӧ����Na2S2O3��CO2�������ӷ���ʽΪ_____________________�����ɵ���������ƴ�Ʒ����_______________ϴ�ӡ�
(3)װ��C�������Ǽ���װ��B��SO2������Ч�ʣ�C���Լ���___________������SO2����Ч�ʵ͵�ʵ��������C����Һ��ɫ�ܿ���ɫ��Ϊ��ʹSO2������������ȫ���ڲ��ı�B����ҺŨ�ȡ�����������£����˼�ʱ���跴Ӧ���⣬���ɲ�ȡ�ĺ�����ʩ��__________(д������)��
(4)���������£�S2O32����������������ԭ��Ӧ����SO2����д��Na2S2O3�����ᷴӦ�����ӷ���ʽ��_______________________��
(5)��Ʒ��Na2S2O3��5H2O�����������IJⶨ
��ȡ6g��Ʒ����250mL����Һ���á���ȡ25mL 0.01 mol/L K2Cr2O7��Һ����ƿ�У�Ȼ����������KI��Һ���ữ�ͼ��ε�����Һ�����������Ƶ�Na2S2O3��Һ�ζ����յ㣬��Na2S2O3��Һ25mL����Ʒ��Na2S2O3��5H2O����������Ϊ________________��(��֪Cr2O72����6I����14H����2Cr3����3I2��7H2O��I2��2S2O32����2I����S4O62��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ԫ��W��X��Y��Z��ԭ������������������Y��ZΪ����Ԫ�أ�����Ԫ�صļ����ӵĵ��Ӳ�ṹ����ͬ��p��q�������еIJ���Ԫ����ɵĻ����r��W�ĵ��ʣ�s��Z�ĵ��ʡ���Щ���ʼ��ת����ϵ��ͼ��ʾ������˵������ȷ����
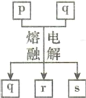
A.ԭ�Ӱ뾶:Y>Z>W>X
B.q�������ӻ�����
C.һ�������£�r��s�ܷ������Ϸ�Ӧ����p
D.p�������ᷴӦ��������������������Һ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ�Dz��ֶ�����Ԫ��ԭ�ӣ�����ĸ��ʾ��������������ԭ�������Ĺ�ϵͼ��˵����ȷ���ǣ� ��
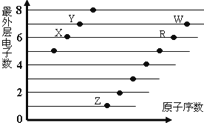
A. X��W�γɵĻ�������ֻ�й��ۼ�
B. X��Z�γɵĻ�������ֻ�����Ӽ�
C. Ԫ�صķǽ����ԣ�X��R��W
D. �����ӵİ뾶��W��R��X
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ҹ������з��ĶԶ��ױ���ɫ�ϳ���Ŀȡ���½�չ����ϳɹ�����ͼ��ʾ��
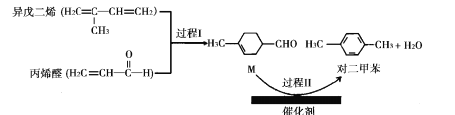
����˵������ȷ����
A.��ϩȩ����������ԭ�ӿ��ܹ�ƽ��B.������ˮ���������ϩ�ͶԶ��ױ�
C.�Զ��ױ��Ķ��ȴ�����6��D.M�ܷ���ȡ�����ӳɣ��Ӿۺ�������Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��W������ԭ��������������Ķ�����Ԫ�أ�W������������ΪX������������һ�룬X��Y��Z��ԭ�Ӱ뾶���μ�С��X��Y��Z��ɵ�һ�ֻ�����(ZXY)2�ĽṹʽΪY��X��Z��Z��X��Y������˵����ȷ����
A.(XY)2��XԪ�صĻ��ϼ�Ϊ+3
B.Y���������Ӧ��ˮ������ǿ��
C.������W(Y3)2��ֻ�������Ӽ�
D.X��Z��ɵĻ������в���������ԭ�Ӷ��ﵽ8�����ȶ��ṹ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com