
����Ŀ����֪ͭ�������A���ṹ��ͼ������ش��������⣺
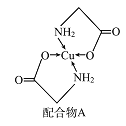


(1)Cu�ļ����Ų�ʽΪ______________ ��
(2)A��������Ԫ��C��N��O�ĵ�һ�������ɴ�С��˳��Ϊ_______________�����е�ԭ�ӵ��ӻ��������Ϊ________��
(3)���就�������(H2NCH2COO-)���ȷֽ�ɲ���CO2��N2��N2��������������Ŀ֮��
��__________��N2O��CO2��Ϊ�ȵ����壬��N2O������Oֻ��һ��N��������N2O�ĵ���ʽΪ_______��
(4)��Cu���£��״��ɱ�����Ϊ��ȩ(HCHO)����ȩ������H CO�ļ���___________��ѡ������������������������С������120������ȩ����ˮ�γ������������ͼ�б�ʾ����___________��
(5)������������ͼ������ʯ�ṹ���ƣ��dz�Ӳ���ϡ���������������B-N��������ԭ����֮��Ϊ___________���ṹ��ѧ����ԭ�����������ʾ�����ڲ���ԭ�ӵ����λ�ã���ͼ���ң�����������ľ����У�Bԭ�ӵ���������ֱ��У�
![]() �ȡ��������������Bԭ������ҵȾ��Nԭ�ӵ��������Ϊ____________________ ��
�ȡ��������������Bԭ������ҵȾ��Nԭ�ӵ��������Ϊ____________________ ��
���𰸡� [Ar]3d104S1 N>O>C SP3�ӻ� 1��2 ![]() ����
����  4��1 ��1/4��1/4��1/4 ��
4��1 ��1/4��1/4��1/4 ��
�����������⿼�����Ԫ��Cu���仯����Ľṹ�������Ų����ӻ����������ṹ�����ʽṹ���й�֪ʶ�㡣����Cu��ԭ�ӽṹ�͵����Ų����ɡ��ӻ���������ӹ��͵�֪ʶ�;���������Լ������й�֪ʶ�������⡣
��1����̬Cuԭ�Ӻ�����29�����ӣ���Χ�����Ų�ʽΪ3d104s1��ȫ�����ṹ���ȶ��������Ų�ʽΪ[Ar]3d104S1
��2��ͬ��������Ԫ�ش����ҵ�һ�����ܳ��������ƣ�����A��͵���A��Ԫ�ط�����Nԭ����Χ�����Ų�Ϊ2s22p3��Ϊ������ṹ�����ȶ���N�ĵ��������C��N��O�ĵ�һ�������ɴ�С��˳��ΪN��O��C����ԭ����4���ӻ����������Ϊ SP3�ӻ�
��3��N2�ĽṹʽΪN��N, ��1��������2������������������������Ŀ��Ϊ1:2��N2O��CO2��Ϊ�ȵ����壬��N2O������Oֻ��һ��N��������N2O�ṹ��CO2���ƣ�������ṹΪN=��=�ϣ�����ʽΪ![]()
��4����ȩ�����У�̼ԭ��Ϊ����ӻ������ӳ�ƽ�������ͣ�����Լ120����������ԭ���йµ��Ӷԣ�����ԭ�����ų����ã����ԣϣãȼ��ǻ��Դ���120�����ʻ����к�ǿ�ĵ縺�ԣ���H2O��H�н�ǿ�ľ��������������γ������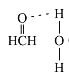
��5����ͼ��֪��һ��Bԭ����4��Nԭ���γ�4��B-N���ۼ���B-N��������ԭ����֮��Ϊ4:1�����ݸ���ԭ�ӵ����λ�ÿ�֪��������������Bԭ������ҵȾ��Nԭ����x��y��z�����������1/4����������������(![]() ��
�� ![]() ��
�� ![]() )
)


 �ƸԹھ��ο���ϵ�д�
�ƸԹھ��ο���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��A��B��C��D��E��F��G����Ԫ�صĺ˵����������������Ԫ�����ڱ���ǰ�����ڵ�Ԫ�ء�����Aԭ���ڻ�̬ʱp���������ҵ縺����ͬ��Ԫ�������ģ�D��Eԭ�Ӻ����M���о�������δ�ɶԵ��ӣ�Gԭ�Ӻ���������������B��ͬ����������������B��E��Ԫ����ɻ�����B2E�ľ���Ϊ���Ӿ��塣C��F��ԭ�Ӿ��������ܲ㣬Cԭ�ӵĵ�һ�����ĵ�����(kJ/mol)�ֱ�Ϊ��578��1817��2745��ll575��C��F���γ�ԭ����Ŀ��Ϊ1:3���۵�Ϊ190���Ļ�����Q��
��1���µĵ��ʾ���Ϊ���������ѻ�ģ�ͣ�����λ��Ϊ ��EԪ�ص������������ӵ����幹���� ��FԪ��ԭ�ӵĺ�������Ų�ʽ�� ��F�ĸ�������A�ļ��⻯���γɵ������ӵĻ�ѧʽΪ .
��2���ԱȽ�B��D�ֱ���F�γɵĻ�������۵�ߵͲ�˵������ ��
��3��A�����γ�ij�ֻ�����ľ����ṹ��ͼ��ʾ ��������٤������ΪNA���û����ᄃ����ܶ�Ϊ a g/cm3���侧���ı߳�Ϊ cm��
��������٤������ΪNA���û����ᄃ����ܶ�Ϊ a g/cm3���侧���ı߳�Ϊ cm��
(4)��1.0l��105Pa��t1��ʱ������Ħ�����Ϊ53.4 L/mol��ʵ����Q����̬�ܶ�Ϊ5.00g/L�����ʱQ�����Ϊ(д��ѧʽ) ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����Ͳ���2017�����ģ�⣨��о�����CO�����ڹ�ҵұ���������ڲ�ͬ�¶���CO��ԭ���ֽ����������ƽ���������lg![]() ���¶�(t)�Ĺ�ϵ��������ͼ������˵����ȷ����
���¶�(t)�Ĺ�ϵ��������ͼ������˵����ȷ����
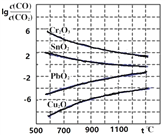
A��ͨ�����߷�Ӧ¯�ĸ߶ȣ��ӳ���ʯ��CO�Ӵ���ʱ�䣬�ܼ���β����CO�ĺ���
B��CO���������ڹ�ҵұ������Cr
C��CO��ԭPbO2�ķ�Ӧ��H��0
D����ҵұ������Cuʱ���������������CO��ת����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ�����Ʊ�����(Cl2)�Ļ�ѧ����ʽ���£�4HCl(Ũ) �� MnO2 ![]() MnCl2 �� Cl2�� ��2 H2O
MnCl2 �� Cl2�� ��2 H2O
��1���õ����ű�ʾ������ת�Ƶķ������Ŀ________________________��
��2��������з�Ӧ��д�����ӷ���ʽ��___________________________��
��3�������������У���������Ԫ����_________����ԭ������___________,�������ͻ�ԭ�������ʵ���֮��Ϊ��__________���÷�Ӧ���������ֵ�����Ϊ��__________________
��4������Ӧ�в���0.5 molCl2����ת�Ƶĵ�����ĿΪ_____����������HCl�����ʵ���___mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ˮ��ѧ2017�쿼ǰģ�⡿һ�ֹ⻯ѧ��صĽṹ����ͼ���������ڱ���Ϳ���Ȼ�������Ƭ��ʱ��AgCl(s) �� AgCl(s)= Ag (s)+Cl(AgCl)��[Cl(AgCl)��ʾ���ɵ���ԭ���������Ȼ�������]������Cl(AgCl)��e������Cl��(aq)��������Դ�Ƴ�����ػ������ظ�����ʼ״̬������˵����ȷ����

A������ʱ��������Y����X
B������ʱ��Pt�缫�����ķ�ӦΪ2Cl����2e��=Cl2
C������ʱ��Cl����Ag�缫�ƶ�
D������ʱ������ܷ�ӦΪ��AgCl(s) + Cu+(aq)��Ag (s) + Cu2+(aq) + Cl��(aq)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1������ͼ��ʾ��������ַ�Ӧ��
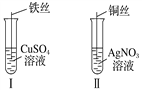
�٢�����˿�Ϲ۲쵽��������______________________��
�ڢ��з�����Ӧ�����ӷ���ʽΪ_______________________��
�۽�Ϣ�ʵ�������֪Fe2����Cu2����Ag������������ǿ������˳��Ϊ_____________��
��2����С�ձ��м���20 mL����ˮ�����������ں����ˮ�е��뼸��FeCl3��Һ�������������Һ�ʺ��ɫ��ֹͣ������
��д���Ʊ�������������Ļ�ѧ����ʽ____________________��
��������������������ε���������ᣬ���ֵ�ʵ������Ϊ��___________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ˮ��ѧ2017��߿�Ѻ���������H2��O2�Ʊ�H2O2��ԭ��Ϊ
�� H2(g)+ A(1)![]() B(1) ��H1 ��O2(g)+B(1)
B(1) ��H1 ��O2(g)+B(1)![]() A(1)+ H2O2(1) ��H2
A(1)+ H2O2(1) ��H2
��֪��A��B��Ϊ�л��������Ӧ�����Է����У�����˵����ȷ����
A����H2>0
B��BΪH2��O2�Ʊ�H2O2�Ĵ���
C����Ӧ��������Ӧ�Ļ�ܴ����淴Ӧ�Ļ��
D��H2(g)+O2(g)![]() H2O2(1) �Ħ�H <0
H2O2(1) �Ħ�H <0
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����д�����з�Ӧ�����ӷ���ʽ��
��1�����������ڴ������ܽ������ݲ�����______________________________________��
��2��CuSO4��Һ��Ba(OH)2��Һ��ϣ�________________________________________��
��3����Ƭ������������Һ��:_________________________________________________��
��һ��ϡ��Һ�������������֣���Һ��ɫ�����壬����ܺ���![]() ��Na+��
��Na+��![]() ��H+��
��H+��![]() ��
��![]() ��Cl�������е������֡�Ȼ�����������·�������ȷ����Щ�����Ƿ���ڡ�
��Cl�������е������֡�Ȼ�����������·�������ȷ����Щ�����Ƿ���ڡ�
����ʯ����Һ������Һʱ����Һ�Ժ�ɫ��
��ȡ2 mL��Һ��BaCl2��Һ��ϡ������м��飬��������˰�ɫ������
�۶Ԣ������õĻ�����־��ú�ȡ���ϲ�����Һ����AgNO3��Һ��ϡ������м��飬����������˰�ɫ������
��ش��������⣺
��1��ԭ��Һ��һ�����ڵ�������__________��һ�������ڵ�������____________��
��2��������ʵ������У��д���IJ�����_________������ţ����Ըô�������ķ����ǣ�Ҫ������ϸ��˵����_________________________________________________________��
��3����ĿǰΪֹ�����ܿ϶���ԭ��Һ���Ƿ���ڵ�������____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и��������������ۻ�ʱ�����˷���������������ȫ��ͬ���� �� ��
A.��������ɱ�����B.���������ۻ��ɱ�����
C.��������������ۻ�D.ʳ���ۻ��ͱ��ڻ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com