
����Ŀ����1����CH3OH�Ϳ���Ϊԭ�ϵ�ȼ�ϵ�أ������ΪKOH��Һ���ش��������⣺
�� OH������____________����
�� ������ӦʽΪ__________________________________��
�� �����pH����______________���������١����䣩��
��2��������ĵ�ض������ʽ��е�⣬����a��b��c��d��e��f�缫��Ϊ���Ե缫��ͨ��һ��ʱ��� e������0.064g����
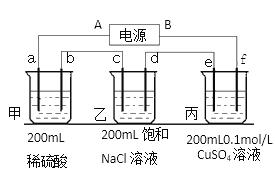
�ٵ�ԴB����____________����
�ڼ׳�a�缫��ӦʽΪ ___________________________________________��
���ҳط�Ӧ��ѧ����ʽ________________________________________________��
���ҳع����ռ�����״�����������Ϊ_____________mL������Һ��pHΪ___________�������£���������Һ����仯��
���𰸡��� CH3OH ��6e-+ 8OH��= 6H2O+CO32�� ��С �� 2H++2e-= H 2�� 2NaCl+2H2O ![]() 2NaOH+Cl2��+H2 �� 44.8 12
2NaOH+Cl2��+H2 �� 44.8 12
��������
��1���ٵ�������������ƶ���
�ڸ�������������Ӧ��
�۵���ܷ�ӦΪ��![]() ��
��
��2������e�����أ���֪e��������ͭ����eΪ��������AΪ������BΪ������a��c��eΪ������b��d��fΪ������
��1���ٵ�������������ƶ�����![]() ������
������
�ڸ�������������Ӧ���״�����������Ӧ����̼������缫��ӦʽΪ��![]() ��
��
�۵���ܷ�ӦΪ��![]() ����Ӧ����������������ҺpH��С��
����Ӧ����������������ҺpH��С��
�ʴ�Ϊ������![]() ����С��
������
��2������e�����أ���֪e��������ͭ����eΪ��������AΪ������BΪ������a��c��eΪ������b��d��fΪ������
��e�����أ���֪e��������ͭ����eΪ��������AΪ������BΪ������
��aΪ�������������������������ŵ������������缫��ӦʽΪ��![]() ��
��
���ҳ���Ϊ���Ե缫����Ȼ�����Һ���ܷ�ӦΪ��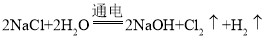 ��
��
�ܸ���e������0.064g��������0.001molCu����ת�Ƶ���0.002mol����Ϊ�Ǵ�����·������ÿ���缫�ĵ�������ͬ������![]() ����֪c������0.001mol
����֪c������0.001mol![]() ��d������0.001mol
��d������0.001mol![]() ���ҳ��в���0.002mol
���ҳ��в���0.002mol![]() �����ҳ��й��ռ���0.002mol���壬0.002mol�����ڱ�״���µ����Ϊ44.8mL���壬
�����ҳ��й��ռ���0.002mol���壬0.002mol�����ڱ�״���µ����Ϊ44.8mL���壬![]() ����pH=12��
����pH=12��
�ʴ�Ϊ������![]() ��
��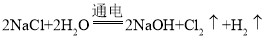 �� 44.8 ��12��
�� 44.8 ��12��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ҹ��涨������ˮ�����ŷŵ��������Ũ��Ϊ0.005mg/L���������ӷ�ˮ�ɲ��û�ѧ���������Իش��������⣺
(1)������[Cd3(PO4)2]�����ܽ�ƽ�ⳣ���ı���ʽKsp=___��
(2)һ���¶��£�CdCO3��Ksp=4.0��10-12��Cd(OH)2��Ksp=3.2��10-14����������ˮ�е��ܽ������ϴ����___��
(3)��ij���ӷ�ˮ�м���Na2S����S2-Ũ�ȴﵽ7.9��10-8mol/Lʱ��ˮ����Cd2+Ũ��Ϊ___mol/L��(��֪��Ksp(CdS)=7.9��1027)����ʱ�Ƿ����ˮԴ����______(����������������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һ��
��1�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ����������������Ϊ__mL���ζ��յ�ʱ�������ǣ����������һ������ʱ����ƿ����Һ��ɫ��___����30s���ָ�ԭɫ��

��2��ijѧ����������ʵ��ֱ��¼�й��������±���
�ζ����� | ������������ ��Һ�����/mL | 0.1000mol��L��1��������/mL | ||
�ζ�ǰ�̶� | �ζ���̶� | ��Һ��� | ||
��һ�� | 25.00 | 0.00 | 26.11 | 26.11 |
�ڶ��� | 25.00 | 1.56 | 30.30 | 28.74 |
������ | 25.00 | 0.22 | 26.31 | 26.09 |
��ѡ�����к����������������������Һ���ʵ���Ũ��(����������4λ��Ч����)��c(NaOH)=__mol��L��1��
��3�����ڴ��������ʹ��������������������Һ��Ũ��ƫ�ߵ���__(����ĸ)��
A.�ζ�ǰ�ζ����������ݣ��ζ�����ʧ B.��ʽ�ζ�����ȡNaOH��Һʱ��δ������ϴ����
C.�ζ�ʱ�ﵽ�ζ��յ�ʱ���Ӷ��� D.��ƿȡ��NaOH����Һǰ������ˮϴ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�������Һ�и�����Ũ�ȹ�ϵ��ȷ����
A.pH��12�İ�ˮ��pH��2������������ϣ�c(Cl��)>c(NH4+)>c(H��)>c(OH��)
B.Ũ��Ϊ0.1mol��L��1��̼������Һ��c(Na��)��c(CO32��)��c(HCO3��)��c(H2CO3)
C.0.1mol��L��1��NaHS��Һ������Ũ�ȹ�ϵ��c(OH��)=c(H+)��c(S2��)+c(H2S)
D.������Һ��NaOH��Һ��Ϻ�������Һ�����ԣ�c(Na��)>c(CH3COO��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��PVAc��һ�־��������Ե���֬���ɺϳ���Ҫ�߷��Ӳ���M���ϳ�·�����£�
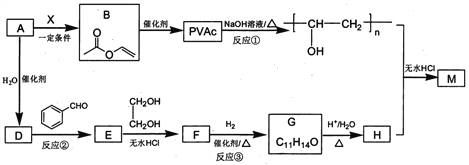
��֪��R��R�@��R�@�@ΪHԭ�ӻ�����
I. R'CHO+ R"CH2CHO![]()
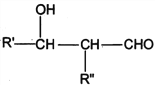
![]()
![]()
II. RCHO+![]()
![]()
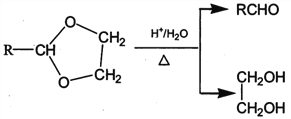
��1����״���£�4.48L��̬��A��������5.2g����A�Ľṹ��ʽΪ___________________��
��2����֪A��BΪ�ӳɷ�Ӧ����X�Ľṹ��ʽΪ_______��B�й����ŵ�������_________��
��3����Ӧ�ٵĻ�ѧ����ʽΪ______________________��
��4��E��ʹ������Ȼ�̼��Һ��ɫ����Ӧ�ڵķ�Ӧ�Լ���������_______________________��
��5����Ӧ�۵Ļ�ѧ����ʽΪ____________________________��
��6����E��F��G��H��ת�������У��Ҷ�����������__________________________��
��7����֪M�������г������⣬��������Ԫ��״�ṹ����M�Ľṹ��ʽΪ_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��
��֪HCO3-��AlO2-��H2O==CO32-��Al(OH)3������������KHCO3��Һ���ϵ��뺬�����ʵ�����KOH��Ba(OH)2��KAlO2�Ļ����Һ�У����ɳ��������ʵ���������KHCO3��Һ����Ĺ�ϵ�ɱ�ʾΪ
A.  B.
B.  C.
C.  D.
D. 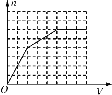
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������ؾ��壨K3[Fe(C2O4)3]��xH2O����һ�ֹ������ϣ���110�����ȫʧȥ�ᾧˮ��Ϊ�ⶨ�þ��������ĺ�����ijʵ��С����������ʵ�飺
�������IJⶨ
����һ������5.00 g�����������ؾ��壬���Ƴ�250 mL��Һ��
�������ȡ������Һ25.00 mL����ƿ�У���ϡH2SO4�ữ���μ�KMnO4��Һ�������ǡ��ȫ�������ɶ�����̼��ͬʱMnO4������ԭ��Mn2+����Ӧ�����Һ�м���һС��п�ۣ���������ɫ�պ���ʧ�����ˣ�ϴ�ӣ������˼�ϴ��������Һ�ռ�����ƿ�У���ʱ��Һ�Գ����ԡ�
����������0.010 mol/L KMnO4��Һ�ζ������������Һ���յ㣬����KMnO4��Һ20.02 mL���ζ���MnO4������ԭ��Mn2+���ظ���������������������ζ�����0.010 mol/L KMnO4��Һ�ֱ�Ϊ19.98 mL��20.00 mL����ش��������⣺
��1���ζ������У����������ҺӦʢװ��__________�ζ����У����ʽ����ʽ������
��2�������ӷ���ʽ��ʾ��������漰������ػ�ѧ��Ӧ��________________�� Zn + 2Fe3+ = 2Fe2+ + Zn2+��
��3���������еζ��յ���ж���____________________��
��4���ڲ�����У��������KMnO4��Һ�������������õ�������____________���ڲ������У����ζ�ǰ���Ӷ������ζ����Ӷ��������õ�������__________����ѡ�ƫ�͡�����ƫ�ߡ��������䡱��
��5��ʵ���øþ�����������������Ϊ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ܱ������н�2molNaHCO3(s)��һ����Na2O2��ϣ��ڼ��������������ַ�Ӧ��150���������������2����֣���Ӧ���������ʵ�����n����ȡֵ��Χ��(����)
A. n��1 B. 1��n��2 C. 2��n��4 D. n��4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������A��B��C��D��E��F ԭ�������������������Ԫ�أ�����λ��Ԫ�����ڱ���ǰ�����ڡ�BԪ�غ���3 ���ܼ����Қ����ܼ������ĵ�������ͬ��D��ԭ�Ӻ�����8���˶�״̬��ͬ�ĵ��ӣ�EԪ����FԪ�ش���ͬһ�������ڵ��壬���ǵ�ԭ���������3����EԪ�صĻ�̬ԭ����4��δ�ɶԵ��ӡ���ش��������⣺
��1����д��D��̬�ļ۲�����Ų�ͼ��_____��
��2������˵����ȷ����_____��
A�������������Է��������ȶ�����̼�����Էе�SiO2��CO2
B���縺��˳��C��N��O��F
C��N2��COΪ�ȵ����壬�ṹ���ƣ���ѧ��������
D���ȶ��ԣ�H2O��H2S��ԭ����ˮ���Ӽ�������
��3��ij��������F(��)(���ʾ���ϼ�Ϊ��1)����γ���ͼ��ʾ�����ӣ���������̼ԭ�ӵ��ӻ���ʽ��______��
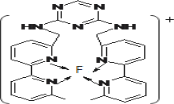
��4����֪(BC)2��ֱ���Է��ӣ����жԳ��ԣ��ҷ�����ÿ��ԭ������㶼�ﵽ8�����ȶ��ṹ����(BC)2�������������ĸ�����Ϊ______��
��5��CԪ����ۺ���������������ǿ�������ԭ����______��
��6��Fe3O4�����У�O2-���ظ����з�ʽ��ͼ��ʾ�������з�ʽ�д���������1��3��6��7��O2-Χ�ɵ����������϶��3��6��7��8��9��12��O2-Χ�ɵ����������϶��Fe3O4����һ���Fe3+��������������϶�У���һ��Fe3+��Fe2+��������������϶�У���Fe3O4�����У����������϶����O2-��֮��Ϊ_____����_____%�����������϶û����������ӡ�Fe3O4��������8��ͼʾ�ṹ��Ԫ�������ܶ�Ϊ5.18g/cm3����þ�������a=______pm��(д���������ʽ)
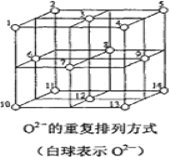
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com