
����Ŀ��I.��ͼ��ʾΪ�������������IJ��ֽṹ��
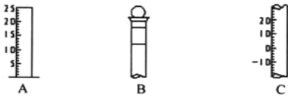
��1����д���������������ƣ�A__________��B____________;C_____________��
��2������ B ʹ��ǰ����______________________��
��3������ B �ϱ����__________________(�����)��
������ ���¶� �ۿ̶��� ��Ũ�� ���ݻ�
II�� ij��ɫ��ҵ��ˮ�п��ܺ���Na+��Mg2+��Al3+��Cl-��SO42-�еļ������ӡ�
a��ȡ�����÷�ˮ���Թ��У�����������Ba(NO3)2��Һ��ϡ���ᣬ������ɫ��������ַ�Ӧ����ˣ�����Һ�м���AgNO3��Һ����������
b.��ȡ10mL�÷�ˮ���Թ��У��μ�NaOH��Һ�Ȳ�����ɫ�������ֳ����ܽ⡣���ɳ��������ʵ��������NaOH�����ʵ�����ϵ����ͼ��ʾ��
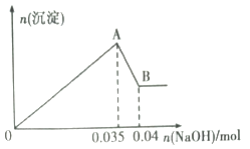
��������ʵ���ͼ�����ݣ�
��1���÷�ˮ��һ�������е�������________________(�����ӷ���)��
��2��д��A��B�����з�����Ӧ�����ӷ���ʽ��_________________��
��3���÷�ˮ�У�c(Al3+)=________________________��
��4����ʵ��������100mL2mol/L��NaOH��Һʱ���õ��IJ����������ձ�������������Ͳ�⣬����Ҫ________(����������)��
�����в���ʹ������ҺŨ��ƫ�����(��д��ĸ)________��
A�������������������
B.��NaOH����ֽ���ϳ���
C.NaOH���ձ����ܽ��δ��ȴ������ת�Ƶ�����ƿ��
D.������ƿת��ʱ��������Һ�����
E��δϴ���ܽ�NaOH���ձ�
F������ʱ���ӿ̶���
G������ƿδ���T����������Һ
���𰸡���Ͳ ����ƿ �¶ȼ� ����Ƿ�©Һ �ڢۢ� Cl- Al(OH)3+OH-=AlO2-+2H2O 0.5mol��L-1 100mL����ƿ����ͷ�ι� AC
��������
a.ȡ0.1mL�÷�ˮ�ڹ��У�����������Ba(NO3)2)��Һ��ϡ���ᣬ������ɫ�������ó���Ϊ���ᱵ����ԭ��Һ�к���SO42-����ַ�Ӧ����ˣ�����Һ�м���AgNO3��Һ����������˵��ԭ��Һ�в�����Cl��b.��ȡ10mL�÷�ˮ�ڹ��У��μ�NaOH��Һ�Ȳ�����ɫ�������ֳ����ܽ⣬�ܽ�ij���Ϊ�������������ܵij���Ϊ������þ����ԭ��Һ��һ������Mg2+��Al3+��(4)��������һ�����ʵ���Ũ�ȵ���Һ�����Ʋ���ȷ��ʹ�õ�����������c=nV��ͨ���жϲ������������ʵ����ʵ���n����Һ���V��Ӱ����������
I.�������Ľṹ�ص���Կ�����A�����������Ͽ̶�������֪����Ͳ��B����ֻ��һ���̶��ߣ���֪������ƿ��C�����������Ͽ̶�����������̶ȣ���̶����»�����ʾΪ��ֵ�Ŀ̶ȣ���֪���¶ȼơ�
II�� (1)���ݷ�����֪���÷�ˮ��һ�������е�������Cl-���ʴ�Ϊ��Cl-��
(2)A��B����Ϊ�����������������Ʒ�Ӧ����ƫ�����ƺ�ˮ����Ӧ�����ӷ���ʽΪ��Al(OH)3+OH-=AlO2-+2H2O���ʴ�Ϊ��Al(OH)3+OH-=AlO2-+2H2O��
(3)���ݷ�ӦAl(OH)3+OH-=AlO2-+2H2O��֪��n[Al(OH)3]=n(NaOH)=0.04mol-0.035mol=0.005mol�����������غ㶨�ɿ�֪��ԭ��Һ�������ӵ����ʵ���Ϊ0.005mol����ԭ��Һ�������ӵ�Ũ��Ϊ��c(Al3+)=0.005mol��0.01L=0.5mol/L���ʴ�Ϊ��0.5molL1��
(4)��ʵ��������100mL molL1��NaOH��Һʱ���õ��IJ����������ձ�������������Ͳ�⣬����100mL��Һ����ѡ�ù��Ϊ100mL������ƿ������ʱ����ʹ�ý�ͷ�ιܣ��ʴ�Ϊ��100mL����ƿ����ͷ�ιܣ�
��A.�������������ƫ��m(��)=m(��)+m(��)���ʳ�������ҩƷ������ƫ�أ������Ƴ�����Һ��Ũ��ƫ��
B.��NaOH����ֽ���ϳ����ᳱ�⣬����������NaOH������ƫС�������Ƴ�����Һ��Ũ��ƫС��
C.NaOH���ձ����ܽ��δ��ȴ��ת�Ƶ�����ƿ�в����ݣ�����Һ��ȴ�����ƫС����Ũ��ƫ��
D.������ƿת��ʱ��������Һ�彦�����ᵼ�����ʵ���ʧ������ҺŨ��ƫС��
E.δϴ���ܽ�NaOH���ձ����ᵼ�����ʵ���ʧ������ҺŨ��ƫС��
F.����ʱ���ӿ̶��ߣ��ᵼ����Һ���ƫ����Ũ��ƫС��
G.ֻҪ�����ʱ��Һ����̶������м��ɣ�����ˮ�����Ⱦ��еĻ��Ǻ�������ģ������ʵ����ʵ�����������Һ���������Ӱ�죬���Ũ����Ӱ�죻
�ʴ�Ϊ��AC��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����
A. CH3CH2CH(CH3)CH3������Ϊ3һ������
B. CH3CH2CH2CH2CH3��CH3CH2CH(CH3)CH3��Ϊͬ��������
C. ![]() ��
��![]() Ϊͬһ����
Ϊͬһ����
D. CH3CH2OH��CH2OHCHOHCH2OH������ͬ�Ĺ����ţ���Ϊͬϵ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�о�С����ij2L�ܱ������м���һ�����Ĺ���A������B��������ӦA(s)+2B(g)![]() D(g)+E(g)����H=Q kJ��mol��1��
D(g)+E(g)����H=Q kJ��mol��1��
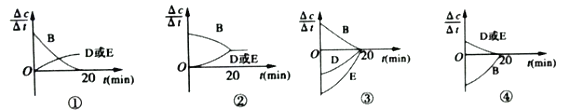
��1�����ڸ÷�Ӧ���ø����ʵķ�Ӧ������ʱ��Ĺ�ϵ���߱�ʾ���£�ʾ��ͼ�е�___________(�����)��ȷ��
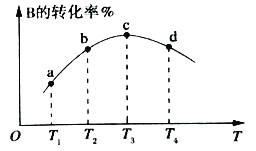
��2�������ܱ��������ȣ�ʵ����B��ת�������¶ȱ仯����ͼ��ʾ����ͼ��֪��Q_________0(��������������С��)��c��v��__________v��(��������������С��������������)��
��3����T��ʱ����Ӧ���е���ͬʱ���ø����ʵ����ʵ��������
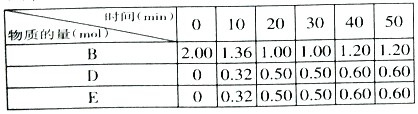
��T��ʱ���÷�Ӧ��ƽ�ⳣ��K=___________��
��30min��ֻ�ı�ijһ��������Ӧ���´ﵽƽ�⣬���ݱ��е������жϸı������������___________(����ĸ���)��
a.ͨ��һ������B b.����һ�����Ĺ���A c.�ʵ���С��������� d.���߷�Ӧ��ϵ�¶�
��ͬʱ����0.2mol B��0.1molD��0.1mol E��������Ӧ�������䣬�÷�Ӧƽ��________�ƶ���(����������������������������)
��ά��������������¶�T�����䣬����������м���1.60molB��0.20molD��0.20molE��0.4molA���ﵽƽ����������B�����ʵ���������___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�¶��£���һ��2 L���ܱ������У�����4 mol A��2 mol B�������·�Ӧ3A(g)��2B(g) ![]() 4C(s)��2D(g)����Ӧһ��ʱ���ﵽƽ�⣬�������1.6 mol C��������˵����ȷ����(����)
4C(s)��2D(g)����Ӧһ��ʱ���ﵽƽ�⣬�������1.6 mol C��������˵����ȷ����(����)
A. �÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ��![]()
B. ��ʱ��B��ƽ��ת������40%
C. �������ϵ��ѹǿ����ѧƽ�ⳣ������
D. ����B��B��ƽ��ת��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����2.3 g��Ͷ��38.8 gˮ�У�������ѧ��Ӧ������ˮ������ʧ1 g��������Һ�����ʵ���������Ϊ�� ��
A. 4.6% B. 7.7% C. 10.0% D. 8.0%
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и�������ֻ��A��B��C���ֵ��ʣ�A����ɫ��ӦΪ��ɫ����Ҫ��G��ʽ�����ں�ˮ�У�F��һ�ֵ���ɫ�Ĺ��壬H��һ�ֳ�������ɫҺ�壬I���������ЧӦ�ġ�Ԫ�ס��������ʼ��ת����ϵͼ���£�
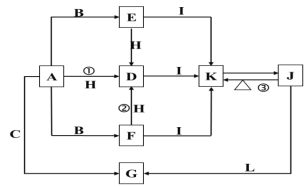
�ش��������⣺
��1��A�ǣ�_____��C�ǣ�_____��L�ǣ�______��I�ǣ�____���ѧʽ����
��2��д����Ӧ�٢ڢ۵Ļ�ѧ��Ӧ����ʽ��
�٣�____________________________________��
�ڣ�____________________________________��
�ۣ�____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D���ֶ�����Ԫ�ص�ԭ�Ӱ뾶���μ�С��D�ֱܷ���A��B��C�γɵ���������ȵķ���X��Y��Z��Cԭ�ӵ����������Ų�Ϊnsnnp2n��E��ԭ������Ϊ29��
(1)A��B��C�ĵ�һ��������С�����˳��Ϊ________(��Ԫ�ط��ű�ʾ)��
(2)X�Ǻ���________��(��Ǽ��ԡ����ԡ�����ͬ)��________���ӡ�
(3)A��һ���⻯�����Է�������Ϊ26��������еĦҼ���м��ļ���֮��Ϊ________��
(4)Y���ӵĿռ乹��Ϊ__________��������ԭ�Ӳ�ȡ________�ӻ���
(5)һ����B��C��ɵĻ�������AC2��Ϊ�ȵ����壬�仯ѧʽΪ________��
(6)Y��һ����Һ�������壬���������Һ����ԭ��_________��
(7)д��E2���ĵ����Ų�ʽ___________________����д��E2����Z��ͨ������Y�õ�����ɫ��Һ�����ӷ�Ӧ����ʽ_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ʵ���;������ǣ� ��
A.��������ˮ��B.�������ƣ���������Ư��
C.̼�����ƣ�θ���кͼ�D.��ʯ�ң�ʳƷ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪�����Ȼ�ѧ����ʽ��
��H2(g)+1/2O2(g)![]() H2O(l) ��H=285.8 kJ��mol1
H2O(l) ��H=285.8 kJ��mol1
��H2(g)+1/2O2(g)![]() H2O(g) ��H=241.8 kJ��mol1
H2O(g) ��H=241.8 kJ��mol1
��C(s)+ 1/2O2(g)![]() CO(g) ��H=110.5 kJ��mol1
CO(g) ��H=110.5 kJ��mol1
��C(s)+O2(g)![]() CO2(g) ��H=393.5 kJ��mol1
CO2(g) ��H=393.5 kJ��mol1
�ش����и����⣺
��1��������Ӧ�����ڷ��ȷ�Ӧ����_______________________��
��2��H2��ȼ����Ϊ________
��3��ȼ��10 g H2����Һ̬ˮ���ų�������Ϊ________��
��4��CO��ȼ����Ϊ________�����Ȼ�ѧ����ʽΪ______________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com