
ΓΨΧβΡΩΓΩAΘ§BΘ§CΘ§DΥΡ÷÷ΕΧ÷ήΤΎ‘ΣΥΊΒΡ‘≠Ή”ΑκΨΕ“ά¥ΈΦθ–ΓΘ§DΡήΖ÷±π”κAΘ§BΘ§C–Έ≥…ΒγΉ”Ήή ΐœύΒ»ΒΡΖ÷Ή”XΓΔYΓΔZΓΘC‘≠Ή”ΒΡΉνΆβ≤ψΒγΉ”≈≈≤ΦΈΣnsnnp2nΓΘEΒΡ‘≠Ή”–ρ ΐΈΣ29ΓΘ
(1)AΘ§BΘ§CΒΡΒΎ“ΜΒγάκΡή”…–ΓΒΫ¥σΒΡΥ≥–ρΈΣ________(”Ο‘ΣΥΊΖϊΚ≈±μ Ψ)ΓΘ
(2)X «Κ§”–________Φϋ(ΧνΓΑΖ«ΦΪ–‘Γ±ΜρΓΑΦΪ–‘Γ±Θ§œ¬Ά§)ΒΡ________Ζ÷Ή”ΓΘ
(3)AΒΡ“Μ÷÷«βΜ·ΈοΒΡœύΕ‘Ζ÷Ή”÷ ΝΩΈΣ26Θ§ΤδΖ÷Ή”÷–ΒΡΠ“Φϋ”κΠ–ΦϋΒΡΦϋ ΐ÷°±»ΈΣ________ΓΘ
(4)YΖ÷Ή”ΒΡΩ’ΦδΙΙ–ΆΈΣ__________Θ§Τδ÷––Ρ‘≠Ή”≤…»Γ________‘”Μ·ΓΘ
(5)“Μ÷÷”…BΘ§CΉι≥…ΒΡΜ·ΚœΈο”κAC2ΜΞΈΣΒ»ΒγΉ”ΧεΘ§ΤδΜ·―ß ΫΈΣ________ΓΘ
(6)Y «“Μ÷÷“Ή“ΚΜ·ΒΡΤχΧεΘ§«κΦρ ωΤδ“Ή“ΚΜ·ΒΡ‘≠“ρ_________ΓΘ
(7)–¥≥ωE2ΘΪΒΡΒγΉ”≈≈≤Φ Ϋ___________________Θ§≤Δ–¥≥ωE2ΘΪ‘ΎZ÷–Ά®»κΉψΝΩYΒΟΒΫ…νάΕ…Ϊ»ή“ΚΒΡάκΉ”Ζ¥”ΠΖΫ≥Χ Ϋ_______ΓΘ
ΓΨ¥πΑΗΓΩC<O<N ΦΪ–‘ Ζ«ΦΪ–‘ 3:2 »ΐΫ«ΉΕ–Έ sp3 N2O Α±ΤχΖ÷Ή”Φδ¥φ‘Ύ«βΦϋΘ§Ζ–ΒψΗΏΕχ»ί“Ή“ΚΜ· 1s22s22p63s23p63d9 Cu2++4NH3H2O=[Cu(NH3)4]2++4H2O
ΓΨΫβΈωΓΩ
AΓΔBΓΔCΓΔDΥΡ÷÷ΕΧ÷ήΤΎ‘ΣΥΊΒΡ‘≠Ή”ΑκΨΕ“ά¥ΈΦθ–ΓΘ§DΡήΖ÷±π”κAΘ§BΘ§C–Έ≥…ΒγΉ”Ήή ΐœύΒ»ΒΡΖ÷Ή”XΓΔYΓΔZΘ§ΥΒΟςDΈΣ«β‘ΣΥΊΘ§“ρΈΣs≤ψ÷ΜΡή «2ΗωΒγΉ”Θ§Υυ“‘n=2Θ§Φ¥C‘≠Ή”ΒΡΉνΆβ≤ψΒγΉ”≈≈≤ΦΈΣ2s22p4Θ§ΈΣ―θ‘ΣΥΊΘ§‘ρAΈΣΧΦΘ§BΈΣΒΣΘ§‘ρ–Έ≥…ΒΡ«βΜ·ΈοΖ÷±πΈΣΦΉΆιΓΔΑ±ΤχΚΆΥ°ΓΘEΒΡ‘≠Ή”–ρ ΐΈΣ29Θ§ΈΣΆ≠‘ΣΥΊΓΘ
(1)Ά§÷ήΤΎΥφΉ≈‘≠Ή”–ρ ΐ‘ω¥σ‘ΣΥΊΒΡΒΎ“ΜΒγάκΡή≥ ‘ω¥σ«ς ΤΘ§ΒΪΒΣ‘ΣΥΊΒΡ2pΡήΦΕ»ίΡ…3ΗωΒγΉ”Θ§ΈΣΑκ¬ζΈ»Ε®Ή¥Χ§Θ§ΡήΝΩΫœΒΆΘ§ΒΎ“ΜΒγάκΡή”…–ΓΒΫ¥σΒΡΥ≥–ρΈΣC<O<NΓΘ(2)X «ΦΉΆιΘ§Κ§”–ΦΪ–‘ΦϋΘ§ΈΣ’ΐΥΡΟφΧεΫαΙΙΘ§Ζ÷Ή”÷–’ΐΗΚΒγΚ…÷Ί–Ρ÷ΊΚœΘ§ τ”ΎΖ«ΦΪ–‘Ζ÷Ή”ΓΘ(3)AΒΡ“Μ÷÷«βΜ·ΈοΒΡœύΕ‘Ζ÷Ή”÷ ΝΩΈΣ26Θ§ΗΟΖ÷Ή”ΈΣ““»≤Θ§ΤδΫαΙΙ ΫΈΣ![]() Θ§ΧΦ«βΒΞΦϋΈΣΠ“ΦϋΘ§ΧΦΧΦ»ΐΦϋΚ§”–“ΜΗωΠ“ΦϋΚΆ2ΗωΠ–ΦϋΘ§Ι ΤδΖ÷Ή”÷–ΒΡΠ“ΦϋΚΆΠ–ΦνΒΡΦϋ ΐ÷°±»ΈΣ3:2ΓΘ(4)YΖ÷Ή”ΈΣΑ±ΤχΘ§Ω’ΦδΙΙ–ΆΈΣ»ΐΫ«ΉΕ–ΈΘ§Τδ÷––Ρ‘≠Ή”ΒΣ‘≠Ή”Φέ≤ψΒγΉ”Ε‘ ΐ=3+Θ®5-1ΓΝ3Θ©/2=4Θ§≤…»Γsp3‘”Μ·ΓΘ(5)“Μ÷÷”…ΒΣΚΆ―θΉι≥…ΒΡΜ·ΚœΈο”κΕΰ―θΜ·ΧΦΜΞΈΣΒ»ΒγΉ”ΧεΘ§ΤδΜ·―ß ΫΈΣN2OΓΘ(6)Α±ΤχΖ÷Ή”Φδ¥φ‘Ύ«βΦϋΘ§Ζ–ΒψΗΏΕχ»ί“Ή“ΚΜ·ΓΘ (7)Ά≠άκΉ”ΒΡΒγΉ”≈≈≤Φ Ϋ1s22s22p63s23p63d9Θ§Ά≠άκΉ”‘ΎΥ°÷–Ά®»κΉψΝΩΑ±ΤχΒΟΒΫ…νάΕ…Ϊ»ή“ΚΒΡάκΉ”Ζ¥”ΠΖΫ≥Χ ΫΈΣCu2++4NH3H2O=[Cu(NH3)4]2++4H2OΓΘ
Θ§ΧΦ«βΒΞΦϋΈΣΠ“ΦϋΘ§ΧΦΧΦ»ΐΦϋΚ§”–“ΜΗωΠ“ΦϋΚΆ2ΗωΠ–ΦϋΘ§Ι ΤδΖ÷Ή”÷–ΒΡΠ“ΦϋΚΆΠ–ΦνΒΡΦϋ ΐ÷°±»ΈΣ3:2ΓΘ(4)YΖ÷Ή”ΈΣΑ±ΤχΘ§Ω’ΦδΙΙ–ΆΈΣ»ΐΫ«ΉΕ–ΈΘ§Τδ÷––Ρ‘≠Ή”ΒΣ‘≠Ή”Φέ≤ψΒγΉ”Ε‘ ΐ=3+Θ®5-1ΓΝ3Θ©/2=4Θ§≤…»Γsp3‘”Μ·ΓΘ(5)“Μ÷÷”…ΒΣΚΆ―θΉι≥…ΒΡΜ·ΚœΈο”κΕΰ―θΜ·ΧΦΜΞΈΣΒ»ΒγΉ”ΧεΘ§ΤδΜ·―ß ΫΈΣN2OΓΘ(6)Α±ΤχΖ÷Ή”Φδ¥φ‘Ύ«βΦϋΘ§Ζ–ΒψΗΏΕχ»ί“Ή“ΚΜ·ΓΘ (7)Ά≠άκΉ”ΒΡΒγΉ”≈≈≤Φ Ϋ1s22s22p63s23p63d9Θ§Ά≠άκΉ”‘ΎΥ°÷–Ά®»κΉψΝΩΑ±ΤχΒΟΒΫ…νάΕ…Ϊ»ή“ΚΒΡάκΉ”Ζ¥”ΠΖΫ≥Χ ΫΈΣCu2++4NH3H2O=[Cu(NH3)4]2++4H2OΓΘ


 ΧλΧλœρ…œ“Μ±ΨΚΟΨμœΒΝ–¥πΑΗ
ΧλΧλœρ…œ“Μ±ΨΚΟΨμœΒΝ–¥πΑΗ –Γ―ß…ζ10Ζ÷÷””Π”ΟΧβœΒΝ–¥πΑΗ
–Γ―ß…ζ10Ζ÷÷””Π”ΟΧβœΒΝ–¥πΑΗ
| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ‘Ύ≤ΜΆ§«ιΩωœ¬≤βΒΟA(g)ΘΪ3B(g)![]() 2C(g)ΘΪ2D(g)ΒΡœ¬Ν–Ζ¥”ΠΥΌ¬ Θ§Τδ÷–Ζ¥”ΠΥΌ¬ Ήν¥σΒΡ «
2C(g)ΘΪ2D(g)ΒΡœ¬Ν–Ζ¥”ΠΥΌ¬ Θ§Τδ÷–Ζ¥”ΠΥΌ¬ Ήν¥σΒΡ «
A. Π‘(D)=0.01 molL-1s-1 B. Π‘(C)=0.5 molL-1min-1
C. Π‘(B)=0.6 molL-1min-1 D. Π‘(A)=0.2molL-1min-1
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩœ¬Ν–Έο÷ ÷–Θ§Φ»Ρή”κ«ΩΥαΖ¥”ΠΘ§”÷ΡήΗζ«ΩΦνΖ¥”ΠΒΡ «Θ® Θ©
ΔΌ![]() ΔΎAl ΔέAl2O3Δή
ΔΎAl ΔέAl2O3Δή![]() Δί
Δί![]()
A.ΔΌΔΎΔέΔήΔίB.ΔΎΔέΔήC.ΔΌΔέΔήD.ΔΌΔΎΔέΔή
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩI.»γΆΦΥυ ΨΈΣ≥ΘΦϊ≤ΘΝß“«ΤςΒΡ≤ΩΖ÷ΫαΙΙΘΚ
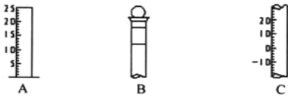
Θ®1Θ©«κ–¥≥ωΥυΝ–“«ΤςΒΡΟϊ≥ΤΘΚA__________Θ§B____________;C_____________ΘΜ
Θ®2Θ©“«Τς B Ι”Ο«Α±Ί–κ______________________ΓΘ
Θ®3Θ©“«Τς B …œ±ξΦ«”–__________________(Χν–ρΚ≈)ΘΜ
ΔΌ÷ ΝΩ ΔΎΈ¬Ε» ΔέΩΧΕ»œΏ Δή≈®Ε» Δί»ίΜΐ
IIΘ° Ρ≥Έό…ΪΙΛ“ΒΖœΥ°÷–Ω…ΡήΚ§”–Na+ΓΔMg2+ΓΔAl3+ΓΔCl-ΓΔSO42-÷–ΒΡΦΗ÷÷άκΉ”ΓΘ
aΘ°»Γ…Ό–μΗΟΖœΥ°”Ύ ‘Ιή÷–Θ§Φ”»κΉψΝΩΒΡBa(NO3)2»ή“ΚΚΆœΓœθΥαΘ§≤ζ…ζΑΉ…Ϊ≥ΝΒμΘ§≥δΖ÷Ζ¥”ΠΚσΙΐ¬ΥΘ§œρ¬Υ“Κ÷–Φ”»κAgNO3»ή“ΚΈό≥ΝΒμ≤ζ…ζΓΘ
b.Νμ»Γ10mLΗΟΖœΥ°”Ύ ‘Ιή÷–Θ§ΒΈΦ”NaOH»ή“Κœ»≤ζ…ζΑΉ…Ϊ≥ΝΒμΘ§Κσ≤ΩΖ÷≥ΝΒμ»ήΫβΓΘ…ζ≥…≥ΝΒμΒΡΈο÷ ΒΡΝΩΥφΦ”»κNaOHΒΡΈο÷ ΒΡΝΩΙΊœΒ»γœ¬ΆΦΥυ ΨΓΘ
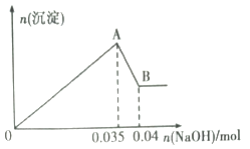
ΗυΨί…œ ω Β―ιΚΆΆΦ÷– ΐΨίΘΚ
Θ®1Θ©ΗΟΖœΥ°÷–“ΜΕ®≤ΜΚ§”–ΒΡάκΉ””–________________(ΧνάκΉ”ΖϊΚ≈)ΘΜ
Θ®2Θ©–¥≥ωAΓζBΙΐ≥Χ÷–ΖΔ…ζΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΘΚ_________________ΘΜ
Θ®3Θ©ΗΟΖœΥ°÷–Θ§c(Al3+)=________________________ΘΜ
Θ®4Θ©ΔΌ Β―ι “≈δ÷Τ100mL2mol/LΒΡNaOH»ή“Κ ±Θ§”ΟΒΫΒΡ≤ΘΝß“«Τς≥ΐ…’±≠ΓΔ≤ΘΝßΑτΓΔΝΩΆ≤ΆβΘ§ΜΙ–η“Σ________(Χν“«ΤςΟϊ≥Τ)ΓΘ
ΔΎœ¬Ν–≤ΌΉς ΙΥυ≈δ»ή“Κ≈®Ε»ΤΪ¥σΒΡ «(Χν–¥Ή÷ΡΗ)________ΓΘ
AΘ°≥ΤΝΩ”ΟΝΥ…ζ–βΒΡμά¬κ
B.ΫΪNaOHΖ≈‘Ύ÷Ϋ’≈…œ≥ΤΝΩ
C.NaOH‘Ύ…’±≠÷–»ήΫβΚσΘ§Έ¥ά以آΝΔΦ¥ΉΣ“ΤΒΫ»ίΝΩΤΩ÷–
D.Άυ»ίΝΩΤΩΉΣ“Τ ±Θ§”–…ΌΝΩ“ΚΧεΦζ≥ω
EΘ°Έ¥œ¥Β”»ήΫβNaOHΒΡ…’±≠
FΘ°Ε®»ί ±―ω ”ΩΧΕ»œΏ
GΘ°»ίΝΩΤΩΈ¥Η…‘οΦ¥”Οά¥≈δ÷Τ»ή“Κ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩœ¬Ν–ΒγάκΖΫ≥Χ Ϋ ι–¥¥μΈσΒΡ «
A. MgCl2ΘΫMg2++2Cl B. NaOHΘΫNa++O2+H+
C. HClΘΫH+ +Cl D. K2SO4ΘΫ2K++SO42
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ”–Έ¥÷Σ≈®Ε»ΒΡBa(OH)2ΚΆNaOHΜλΚœ»ή“ΚΘ§ΝΩ»ΓΥΡΖίΗΟ»ή“ΚΖ÷±πΆ®»κΒ»ΝΩΒΡCO2Θ®“―’έΥψ≥…±ξΉΦΉ¥Ωωœ¬ΒΡΧεΜΐΘ§≤ΜΩΦ¬«CO2‘ΎΥ°÷–ΒΡ»ήΫβΘ©Θ§…ζ≥…≥ΝΒμΒΡΈο÷ ΒΡΝΩ»γœ¬±μΘΚ
Β―ι–ρΚ≈ | Δώ | Δρ | Δσ | Δτ |
CO2ΧεΜΐ(mL) | 2352 | 2352 | 2352 | 2352 |
―υΤΖΧεΜΐ(mL) | 20.0 | 30.0 | 40.0 | 50.0 |
≥ΝΒμΈο÷ ΒΡΝΩ(ΓΝ10-2mol) | 1.50 | 4.50 | 6.00 | 7.50 |
Θ®1Θ©ΗΟΜλΚœ»ή“Κ÷–Ba(OH)2ΒΡΈο÷ ΒΡΝΩ≈®Ε»=_____________ΓΘ
Θ®2Θ© Β―ιΔσΉνΚσΥυΒΟ»ή“Κ÷–ΧΦΥαΡΤΒΡΈο÷ ΒΡΝΩ=___________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ≥ΘΈ¬œ¬Θ§ΫΪCl2ΜΚ¬ΐΆ®»κΥ°÷–÷Ν±ΞΚΆΘ§»ΜΚσœρΥυΒΟ±ΞΚΆ¬»Υ°÷–ΒΈΦ”0.10 molΓΛLΘ≠1ΒΡNaOH»ή“ΚΘ§’ϊΗωΙΐ≥Χ÷–»ή“ΚpH±δΜ·ΒΡ«ζœΏ»γΆΦΥυ ΨΓΘœ¬Ν––π ω÷–’ΐ»ΖΒΡ «

A. ΒψΔΌΥυ Ψ»ή“Κ÷–ΘΚc(H+)=c(ClΘ≠)+c(HClO)+c(OHΘ≠)
B. ΒψΔΎΥυ Ψ»ή“Κ÷–ΘΚc(H+)ΘΨc(ClΘ≠)ΘΨc(ClOΓΣ)ΘΨc(HClO)
C. ΒψΔέΥυ Ψ»ή“Κ÷–ΘΚc(Na+)=2c(ClOΘ≠)+c(HClO)
D. ΒψΔήΥυ Ψ»ή“Κ÷–ΘΚc(Na+)>c(ClOΘ≠)>c(ClΘ≠)>c(HClO)
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ…ηNAΈΣΑΔΖϋΦ”Β¬¬ό≥Θ ΐΒΡ÷ΒΘ§œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «
A. 56 g Fe ”κ1mol Cl2≥δΖ÷Ζ¥”ΠΘ§ΉΣ“ΤΒΡΒγΉ” ΐΈΣ3NA
B. Ζ÷Ή” ΐΈΣ 0.1NAΒΡCH4ΚΆNH3ΜλΚœΤχΧεΘ§Κ§”–ΒΡΙ≤”ΟΒγΉ”Ε‘ ΐΈΣ0.4NA
C. 25Γφ ±Θ§1L pH=12ΒΡBa(OH)2»ή“Κ÷–Κ§”–0.02NA ΒΡOHΘ≠
D. ≥ΘΈ¬≥Θ―Ιœ¬Θ§22 g D3O+÷–Κ§”–10NAΗωΒγΉ”
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩœ¬Ν–Έο÷ ΉΣΜ·‘ΎΗχΕ®ΧθΦΰœ¬ΟΩ“Μ≤ΫΕΦΡή Βœ÷ΒΡ «
A. Si![]() SiO2
SiO2![]() H2SiO3
H2SiO3
B. Mg(OH)2![]() MgCl2(aq)
MgCl2(aq) ![]() Mg
Mg
C. Al2O3![]() A1Cl3(aq)
A1Cl3(aq) ![]() ΈόΥ°AlCl3
ΈόΥ°AlCl3
D. CH3CHO![]() CH3COOH
CH3COOH![]() CH3COOCH3
CH3COOCH3
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΙζΦ ―ß–Θ”≈―Γ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com