
����Ŀ�������������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�������ͼΪʵ������ȡ����ˮ��ʵ��װ�á�
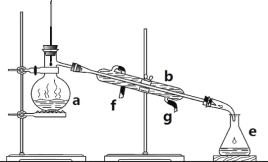
��1��д���������������ƣ�b.__��e___��
��2�������ø�װ�÷������Ȼ�̼�;ƾ��Ļ�����ȱ�ٵ�������___���������������������ʵ�飬�¶ȼ�ˮ�����λ����___��������ˮ��__����f��g����ͨ�룬��һ������������a___�����ܻ��ܣ�ֱ�Ӽ��ȡ�
��3��ʵ��ʱa�г�������������ˮ�⣬�����������__����������___��
���𰸡������� ��ƿ �ƾ��� ������ƿ��֧�ܿ� g ���� ���Ƭ�����ʯ�� ��ֹ����
��������
��1����ͼ�����������÷�����֪�����������ƣ�bΪ�����ܣ�eΪ��ƿ��
��2�����ø�װ�÷������Ȼ�̼�;ƾ��Ļ�����ȡ������Ҫ���ȣ���ȱ�ٵ������Ǿƾ��ƣ��������������������ʵ�飬Ҫ���¶ȼƲ����������������¶ȣ��¶ȼ�ˮ�����λ����������ƿ��֧�ܿڴ���ʵ������У���Ҫͨ��ˮ����������ԭ��������ˮ��g��ͨ�룬��һ������������aΪ������ƿ����ֱ�Ӽ��ȣ�Ҫ��ʯ�������ȡ�
��3������ʵ�����ʱΪ��ֹҺ�����ʱ�ľ�����������������Ƭ�����ʯ������ʵ��ʱa�г�������������ˮ�⣬��������������Ƭ�����ʯ�����������Ƿ�ֹ���С�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ȡa molij�����������������ʹ����2a mol O2��ϵ�ȼ������ǡ����ȫ��Ӧ�����ɵ������CO2��ˮ��������Ӧ����������ܶȽϷ�Ӧǰ������![]() (������ͬ�����²ⶨ)������л���ķ���ʽΪ (����)
(������ͬ�����²ⶨ)������л���ķ���ʽΪ (����)
A.C2H4B.C3H6O2C.C2H4O2D.C4H8O2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ۺͻ���̿�Ļ������NaCl��Һ�����������ͼװ���У��������ĵ绯ѧ��ʴʵ�顣�����йظ�ʵ���˵����ȷ���ǣ� ��
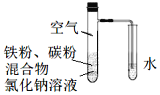
A.���������ĵ缫��ӦʽΪFe�C3e=Fe3+
B.����ʴ�����л�ѧ��ȫ��ת��Ϊ����
C.����̿�Ĵ��ڻ�������ĸ�ʴ
D.��ˮ����NaCl��Һ�������ܷ���������ʴ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2-������ϩ��(����������)��һ����Ҫ��ҽҩ�м��塢�����м��壬�������з����ϳɡ����ȣ���A�Ƶ�E���������£�
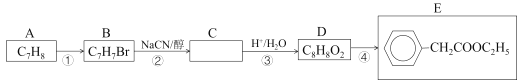
��֪��i.CH3CH2Br+NaCN![]() CH3CH2CN+NaBr
CH3CH2CN+NaBr
ii.CH3CH2CN![]() CH3CH2COOH
CH3CH2COOH
��1����A��������___��D��ͬ���칹���У��ܷ���������Ӧ�ķ����廯�����ж��֣���дһ�ָ���ͬ���칹��Ľṹ��ʽ___��д����Ӧ�ܵĻ�ѧ����ʽ___��
����֪��iii��R��CH2��COOCH2R��+HCOOCH2R��![]() +R����CH2OH
+R����CH2OH
Ȼ��ͨ������·�߿ɵ����ղ�Ʒ��
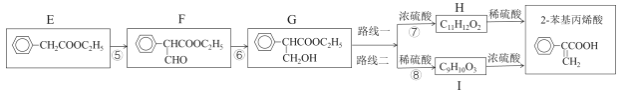
��2������F�Ƿ���ȫת��ΪG���Լ���___����Ӧ�ݵĻ�ѧ����ʽ��___��
��3��·�߶���·��һ��Ȳ�̫���룬������___��
��4����Ʋ��������ºϳ�����ͼ___��

���ϳ�·�߳��õı�ʾ��ʽΪ��A![]() B����
B����![]() Ŀ����
Ŀ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ������(��)Һ�������(Sb2S3���뵼��)��ȡ���װ����ͼ��ʾ�� ����˵���������( )
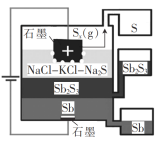
A.Sb����������
B.�������廥�����ܣ��ܶ�Ҳ��ͬ
C.�����ĵ缫��ӦʽΪxS2- - 2xe- = Sx
D.�õ��տ��������¹���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����15�֣�����ˮ������������SMR���Ǵ�ͳ��ȡ������������Ҫ���������й��ռ��ɱ��͵��ŵ㡣
�ش��������⣺
��1����֪1000 Kʱ�����з�Ӧ��ƽ�ⳣ���ͷ�Ӧ�ȣ�
��CH4(g)![]() C(s)+2H2(g) K1=10.2 ��H1
C(s)+2H2(g) K1=10.2 ��H1
��2CO(g)![]() C(s)+CO2(g) K2=0.6 ��H2
C(s)+CO2(g) K2=0.6 ��H2
��CO(g)+H2O(g)![]() CO2(g)+H2(g) K3=1.4 ��H3
CO2(g)+H2(g) K3=1.4 ��H3
��CH4(g)+2H2O(g)![]() CO2(g)+4H2(g) K4 ��H4��SMR��
CO2(g)+4H2(g) K4 ��H4��SMR��
��1000 Kʱ��K4=____________����H4=_________������H1����H2����H3����ʾ����
��2���ڽ��������װ��ǰ����Ҫ��ԭ�����������������ʹ��Ũ��Ϊ0.5 ppm���¡������Ŀ��Ϊ______________��
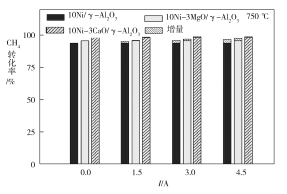
��3����ͼΪ��ͬ�¶������µ���ǿ�ȶ�CH4ת���ʵ�Ӱ�졣��ͼ��֪�������Բ�ͬ��������ͬ�¶������µļ���ˮ������������Ӧ�����Ŵٽ����ã������֪��H4____0������>������<������

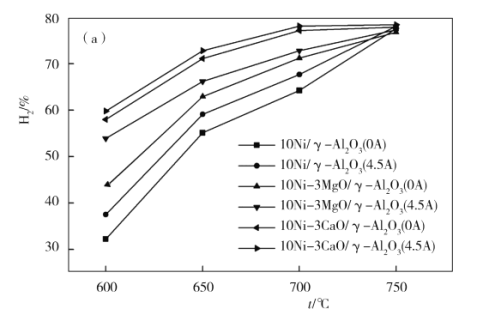
��4����ͼΪ��ͬ�¶�������6Сʱ�ȶ����Ե���ǿ�ȶ�H2���ʵ�Ӱ�졣��ͼ��֪�������¶ȵĽ��ͣ�������H2���ʵ�Ӱ��������____________����������������С����������������600 ��ʱ�����������ִ����е�____________����ͼ�еĴ�����ʾʽ�ش�Ӱ��Ч����Ϊ���������¶ȸ���750 ��ʱ�����۵���ǿ�ȴ�С����������H2����������ͬ����ԭ����______________��
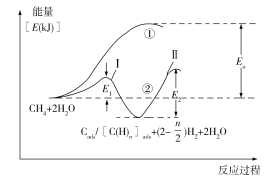
��5���ҹ���ѧ�ҶԼ����ˮ������������Ӧ����Ҳ�����˹㷺�о���ͨ����Ϊ�÷�Ӧ���������С���һ����CH4���ѽ�����H2��̼����̼�����֣�������̼����̼�����֣������ڴ����ϣ���CH4��Cads/[C(H)n]ads+(2�C![]() )H2���ڶ�����̼����̼�����֣���H2O��Ӧ����CO2��H2����Cads/[C(H)n]ads +2H2O��CO2 +(2+
)H2���ڶ�����̼����̼�����֣���H2O��Ӧ����CO2��H2����Cads/[C(H)n]ads +2H2O��CO2 +(2+![]() )H2����Ӧ���̺������仯��ͼ���£����̢�û�мӴ��������̢ڼ�������������̢ٺ͢���H�Ĺ�ϵΪ����_______�ڣ��������������������������������������̢ڷ�Ӧ���ʵ��ǵ�_______������ԭ��Ϊ____________________________��
)H2����Ӧ���̺������仯��ͼ���£����̢�û�мӴ��������̢ڼ�������������̢ٺ͢���H�Ĺ�ϵΪ����_______�ڣ��������������������������������������̢ڷ�Ӧ���ʵ��ǵ�_______������ԭ��Ϊ____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������е�18��Ԫ�ؾ�����Ҫ����;�����ִ���ҵ�б���������
(1)����һ��Ӳ���ࡢ����ʴ��ǿ�Ľ����������ڵ�ƺ��������ָ֡���̬Crԭ���У�����ռ������ܲ�ķ���Ϊ______�����ܲ��Ͼ��е�ԭ�ӹ����Ϊ________��������Ϊ________��
(2)��������Ԫ�صĵ�һ��������ԭ��������������������������ģ�30Zn��31Ga�ĵ�һ�������Ƿ������һ���ɣ�________(����������������)��ԭ����_____________________________________________ (���ǰһ���������������ʿ��Բ���)��
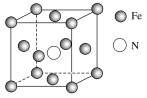
(3)�������A��Ԫ�ؿ��γɶ��������˹��뵼����ϣ��黯��(GaAs)��������һ�֣��侧��ṹ����ͼ��ʾ(��ɫ�����Asԭ��)����GaAs�����У�ÿ��Gaԭ����________��Asԭ����������ͬһ��Gaԭ��������Asԭ�ӹ��ɵĿռ乹��Ϊ________��
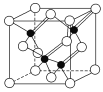
��4�����Ķ��ֻ������Ϊ���Բ��ϣ�������������һ�֣�ij�������ľ����ṹ��ͼ��ʾ�������Ļ�ѧʽΪ________���辧���߳�Ϊa cm�������ӵ�����ΪNA���þ�����ܶ�Ϊ________ g��cm��3(�ú�a��NA��ʽ�ӱ�ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�¶��£�wgij���������������г��ȼ�գ���ȼ�ղ��������������Na2O2��Ӧ��������������wg������H2����CO����CO��H2�Ļ���� ��HCHO����CH3COOH����HO-CH2-CH2-OH�У������������
A. �������� B. ֻ���٢ڢ� C. ֻ���ܢ� D. ȫ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ԫ�أ�����A��X��Y��D��EΪ����������Ԫ�أ�Z��GΪ��������Ԫ�أ����ǵ�ԭ����������������ش����⡣
A | Ԫ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�� |
X | Ԫ��ԭ�ӵĺ��� |
Y | ԭ�ӵĵ�һ�����ĵ����ֱܷ��ǣ� |
D | ԭ�Ӻ������� |
E | Ԫ�ص������������������IJ�Ϊ4 |
Z | ��ǰ�������е縺����С��Ԫ�� |
G | �����ڱ��ĵ����� |
��1����֪![]() Ϊ���ӻ����д�������ʽ_______________��
Ϊ���ӻ����д�������ʽ_______________��
��2��X��̬ԭ����������ߵĵ��ӣ���������ڿռ���______������ԭ�ӹ����_____�Σ�![]() �Ŀռ乹��Ϊ__________��
�Ŀռ乹��Ϊ__________��
��3��ijͬѧ����������Ϣ���ƶ�Y��̬ԭ�ӵĺ�������Ų�Ϊ��
![]()
��ͬѧ�����ĵ����Ų�ͼΥ����________________________��
��4��Gλ��Ԫ�����ڱ���_________����ԭ�ӽṹʾ��ͼΪ____________________��
��5��![]() ����ԭ�ӵ��ӻ���ʽΪ_________����ռ乹��Ϊ____________________��
����ԭ�ӵ��ӻ���ʽΪ_________����ռ乹��Ϊ____________________��
��6��ZԪ�صĻ�̬ԭ�Ӽ۵����Ų�ʽΪ_______________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com