
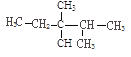
����Ŀ�������������еĻ��ţ���CH3����CH2����![]() ��
��![]() �е�̼ԭ�ӷֱ��Ϊ�����١��塢��̼ԭ�ӣ���Ŀ�ֱ���n1��n2��n3��n4��ʾ�����磺
�е�̼ԭ�ӷֱ��Ϊ�����١��塢��̼ԭ�ӣ���Ŀ�ֱ���n1��n2��n3��n4��ʾ�����磺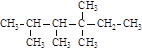 �����У�n1=6��n2=1��n3=2��n4=1���Ը��ݲ�ͬ��������ɽṹ������������(��������)��ԭ�����Ĺ�ϵ��
�����У�n1=6��n2=1��n3=2��n4=1���Ը��ݲ�ͬ��������ɽṹ������������(��������)��ԭ�����Ĺ�ϵ��
(1)������������ԭ����n0��n1��n2��n3��n4֮��Ĺ�ϵ��n0=_________________��
(2)����̼ԭ����֮��Ĺ�ϵΪn1=_________________________________________��
(3)��������n2=n3=n4=1����÷��ӵĽṹ��ʽ����Ϊ(��дһ��)________________��
���𰸡�2(n1��n2��n3��n4)��2��3n1��2n2��n3 n3��2n4��2 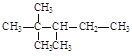
��������
��1��ֻ�в����١���̼ԭ���Ϻ�����ԭ�ӣ������ԭ������Ӧ���Dz����١���̼ԭ���Ϻ��е���ԭ����֮�ͣ����ݲ����١��塢��̼ԭ���ϵ���ԭ��������Ϊ3��2��1��0�������Եó���ԭ����Ϊn0=3n1+2n2+n3�����������е�̼ԭ������Ϊn=n1+n2+n3+n4,�������������ͨʽCnH2n+2��֪����ԭ�ӵ�����Ϊn0=2n+2=2(n1+n2+n3+n4)+2����ȷ�𰸣�2(n1��n2��n3��n4)��2��3n1��2n2��n3��
��2����̼����CH2����ԭ��ֻ��2����ۣ�����ֱ�ӽ������̼���У���̼ԭ��(![]() )����̼���У�����һ����ۣ����������Ҫ����һ��һ�ۻ���(����CH3)����̼ԭ��(
)����̼���У�����һ����ۣ����������Ҫ����һ��һ�ۻ���(����CH3)����̼ԭ��(![]() )����̼���У�����������ۣ����������Ҫ��������һ�ۻ���(����CH3)���ټ�����������������һ�ۻ���(����CH3)�����������CH3����(����̼ԭ����n1)Ϊ����̼ԭ������n3������̼ԭ������n4����2����2������n1=n3+2n4+2����ȷ�𰸣�n1=n3+2n4+2��
)����̼���У�����������ۣ����������Ҫ��������һ�ۻ���(����CH3)���ټ�����������������һ�ۻ���(����CH3)�����������CH3����(����̼ԭ����n1)Ϊ����̼ԭ������n3������̼ԭ������n4����2����2������n1=n3+2n4+2����ȷ�𰸣�n1=n3+2n4+2��
��3����������n2=n3=n4=1����1����̼ԭ�ӣ�����2��������1����̼������1����������̼ԭ������=n2+n3+1+n4+2+2=8�������ķ���ʽ��C8H18������n2=n3=n4=1�ṹ��ʽ�У�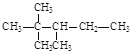 ��
��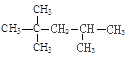 ��
�� ����ȷ�𰸣�
����ȷ�𰸣�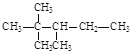 ��
�� ��
�� ��
��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����³������������θ�ס������ס���֭��й�ϰ����ູ֢���и�Ż�¼�������³�����ĺϳ�·�����£�

�ش��������⣺
��1���л���A��������_____��F�й����ŵ������� ____��
��2��̼ԭ���������ĸ���ͬ��ԭ�ӻ����ʱ����̼��Ϊ����̼����³�����ṹʽ��____��������������û����������̼ԭ�ӡ�
��3��A��B�ķ�Ӧ����Ϊ____��
��4��C��D��Ӧ�Ļ�ѧ����ʽΪ____��
��5��X��C��ͬ���칹�壬X�г������ⲻ��������״�ṹ��X����FeCl3��Һ������ɫ��Ӧ������������X �Ľṹ��____�֣����к˴Ź�������Ϊ3��壬�����֮��Ϊ1:2:2��X�Ľṹ��ʽΪ____��
��6���ο��������̣������CH2=CHCH2OH�����ᡢ�״�Ϊ��ʼԭ�Ϻϳ�![]() �ĺϳ���·�����Լ���ѡ��____
�ĺϳ���·�����Լ���ѡ��____
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��a mol H2��b mol C2H2���ܱ������з�Ӧ������ﵽƽ��ʱ������c mol C2H4����ƽ����������ȫȼ������CO2��H2O���������������ʵ���Ϊ
A. (![]() )mol B. (a��3b)mol
)mol B. (a��3b)mol
C. (![]() )mol D. (a��3b��2c)mol
)mol D. (a��3b��2c)mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ȼ�ϵ�ظ���(ȼ��)�缫��Ӧʽ����д
(1)��ȼ�ϵ��
�����Խ���
CH4��1CH4��8 e��+2H2O= 1CO2+8H+
C2H6 ��____________
C2H2 �� ___________
�ڼ��Խ���
CH4��1CH4��8 e��+ 10OH��= 1 CO32��+ 7 H2O
C2H6��1 C2H6��14e�� + 18OH��= 2CO32��+12H2O
C2H2��______________
(2)���ĺ���������ȼ�ϵ��
�����Խ���
CH3OH��1CH3OH��6e��+1H2O = 1CO2+6 H+
C2H5OH�� ______________
CH3OCH3��_____________
�ڼ��Խ���
CH3OH�� 1CH3OH��6e��+8OH ��= 1CO32��+ 6 H2O
C2H5OH�� ___________
CH3OCH3��___________
(3)����̼����Ϊ�����
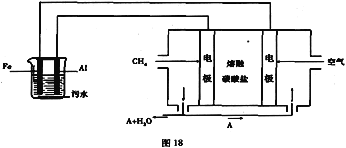
����(CH4)��___________
����(O2)��______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵������ȷ����(����)
A.��״���£�22.4 Lˮ�������ķ�����ԼΪ6.02��1023��
B.1 mol Cl2�к��е�ԭ����ΪNA
C.��״���£�aL�����͵����Ļ���ﺬ�еķ�����ԼΪ![]() ��6.02��1023��
��6.02��1023��
D.���³�ѹ�£�11.2 L CO��������0.5NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪X��Y��Z����Ԫ�ؾ�Ϊ������Ԫ�أ�ԭ���������ε�����Q��WΪǰ30��Ԫ�ء�5��Ԫ�صIJ����ص㣺
Ԫ�� | �ص� |
X | ����̬�⻯��������Σ�ˮ��Һ�Լ��� |
Y | ��̬ԭ�Ӻ����������ܼ��������������������ڴ�����������3�� |
Z | Ԫ��ԭ�ӵ���Χ���Ӳ��Ų�ʽΪnsn-1npn-1 |
Q | ����Ϊ��̫�ս�������Ҳ�С����������֮�ƣ����̬ԭ�Ӵ������2��δ�ɶԵ��� |
W | ԭ��M�ܼ���Ϊȫ����״̬�Һ����δ�ɶԵ���ֻ��һ�� |
��1��XF3������X���ӻ�����Ϊ______���÷��ӵĿռ乹��Ϊ_______��
��2����̬Qԭ�ӵĵ����Ų�ʽΪ_____________________����һ�����ܣ�X______Y���������� ����������=���� ��
��3��X��Y��Z�縺���ɴ�С��˳��Ϊ____________����Ԫ�ط��ű�ʾ����
��4��Na��Y2��ȼ�ղ���ĵ���ʽΪ________________��
��5��Z��X�γɵĻ����ﳣ���������ͻ���ϣ���ѧ�����ȶ����ݴ��Ʋ���Ӧ����_______���壮
��6��WԪ����XԪ���γ�ij�ֻ�����ľ����ṹ��ͼ��ʾ���������Wԭ�ӣ������þ�����ܶ�Ϊ�� g��cm-3����þ����������__________cm3��
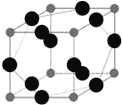
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʵ������������ԭ�����͵���
A. ��ҵ�������ڶ������������ʱ���ø�������ߵ�λʱ��SO3�IJ���
B. �ϳɰ���ҵ��ʹ������ý���������ӿ췴Ӧ����
C. �ñ���ʳ��ˮ����ȥ�������Ȼ�������
D. ����2HI(g)![]() H2(g)+I2(g)����ƽ�����С���������ʹ��ϵ��ɫ����
H2(g)+I2(g)����ƽ�����С���������ʹ��ϵ��ɫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��Ԫ����B(OH)2(K1=5.9��10-2��K2=6.4��10-5)����10mLϡB(OH)2��Һ�еμӵ�Ũ��������Һ��B(OH)2��B(OH)+��B2+��Ũ�ȷ���������ҺPOH[POH=-lgc(OH)-]�仯�Ĺ�ϵ��ͼ������˵����ȷ����
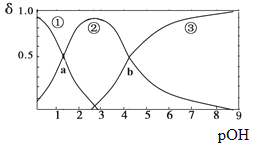
A. ����a����Ӧ�����������Һ�����Ϊ5mL
B. �������������Һ�����Ϊ10mLʱ����c(Cl-)>c(B(OH)+)>c(H+)>c(OH-)>c(B2+)
C. ����b��c(OH)=6.4��10-5
D. �������������Һ�����Ϊ15mLʱ���ڣ�c(Cl-)+c(OH-)= c(B2+)+c(B(OH)+)+ c(H+)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧΪȷ��ij���ķ���ʽ������ܵĽṹ��ʽ����������ʵ�飺��״���£�ȡ0.1molij��������������ȫȼ�գ����ɵĶ�����̼���Ϊ22.4L��ͬʱ�õ�16.2gˮ��
��1��������Ħ������Ϊ______________��
��2�������ķ���ʽΪ____________________��
��3��������һ�ֿ��ܵĽṹΪ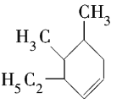 ����ϵͳ����Ϊ___________��
����ϵͳ����Ϊ___________��
��4�� ����______________��ѡ����ţ���
����______________��ѡ����ţ���
a.����b.����c.������d.��������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com